AskBio Commences Phase 2 Clinical Study of GenePHIT in Heart Failure Treatment
Asklepios BioPharmaceutical, Inc., an entirely owned gene therapy entity operating autonomously under the umbrella of Bayer AG, revealed the commencement of GenePHIT, a second-phase clinical study which will evaluate the efficacy of AB-1002 (also known as NAN-101), in addressing the condition of congestive heart failure.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The clinical study known as GenePHIT involves a sophisticated, double-masked, placebo-matched, stratified, multi-center research to investigate the tolerance and effectiveness of a singular intracoronary administration of AB-1002 in mature individuals who have non-ischemic cardiomyopathy. These subjects are categorized under Class III Heart Failure by the New York Heart Association and have maintained clinical stability for a minimum duration of four weeks.
The progression of AB-1002 to a Phase 2 clinical trial is a critical development in the exploration of this innovative gene treatment for CHF. If the trial yields positive results, it has the potential to move this crucial experimental treatment forward, addressing the needs of patients with significant medical deficiencies.
The GenePHIT study aims to recruit between 90 and 150 adult participants whose left ventricular ejection fraction ranges from 15% to 35%. These individuals persistently experience heart failure symptoms despite adhering to recommended treatment protocols. The main gauge for effectiveness after one year involves a composite win ratio derived from various significant clinical evaluations.
“AskBio enthusiastically embarks on the GenePHIT study under the supervision of Roger Hajjar, MD, the Scientific Chair for CHF, and Lothar Roessig, MD, the Head for the Integrated Product Team CHF,” proclaimed Jude Samulski, PhD, a founding member and Chief Scientific Executive at AskBio. “We are hopeful this investigation will clarify the efficacy of AB-1002 as a potential remedy for one of the most debilitating global illnesses. We are eager to gain further insight into this vital experimental treatment for cardiac gene therapy.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
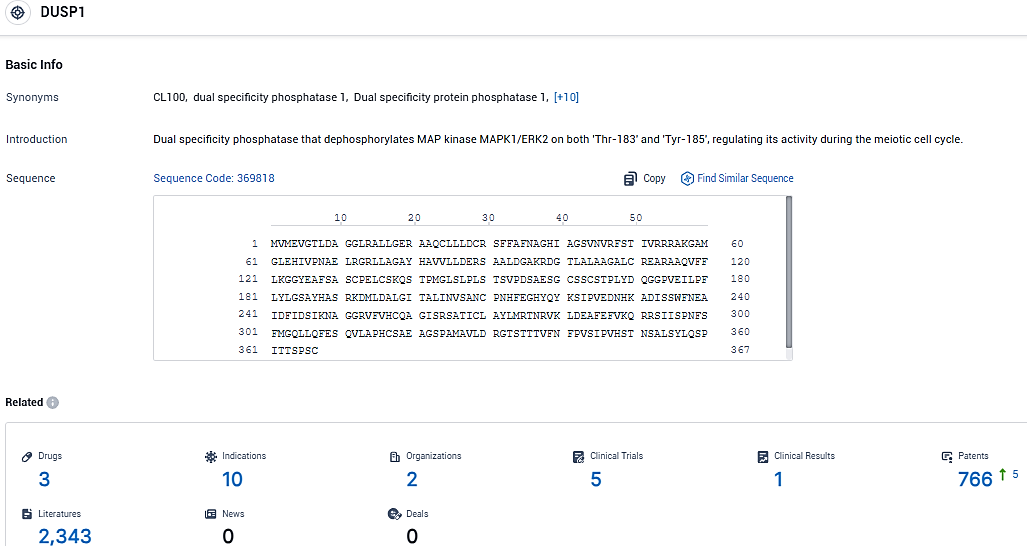
According to the data provided by the Synapse Database, As of January 12, 2024, there are 3 investigational drugs for the DUSP1 target, including 10 indications, 2 R&D institutions involved, with related clinical trials reaching 5, and as many as 766 patents.
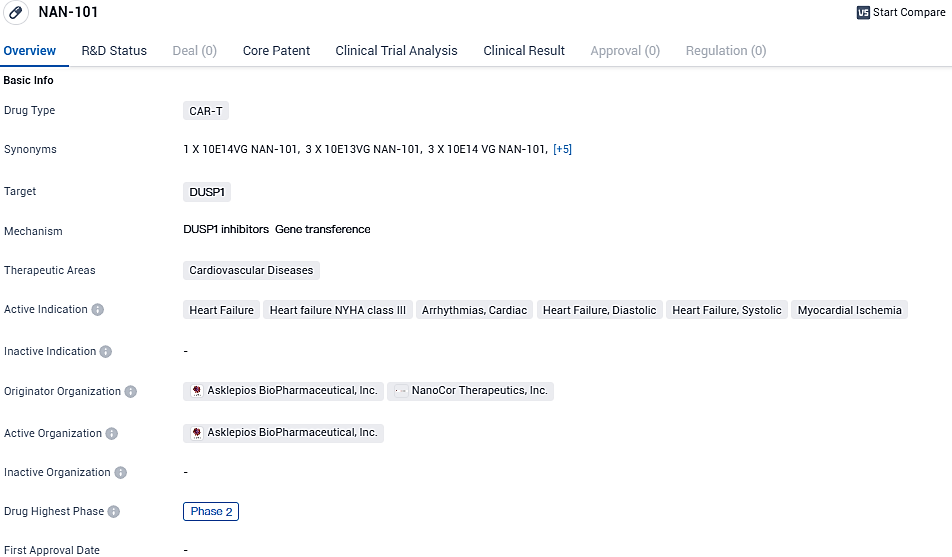
NAN-101 is a CAR-T drug being developed by Asklepios BioPharmaceutical, Inc. and NanoCor Therapeutics, Inc. for the treatment of cardiovascular diseases. It targets DUSP1 and is currently in Phase 2 of clinical trials. The drug shows potential for addressing conditions such as heart failure, arrhythmias, and myocardial ischemia. Further research and testing will be necessary to determine its ultimate efficacy and safety.