An analysis of Lifileucel's R&D progress and its clinical results presented at the 2023 ESMO_IO Annual Meeting
The large proportion of patients (pts) with advanced (unresectable or metastatic) melanoma resistant to immune checkpoint inhibitors (ICI) define a significant unmet need. Lifileucel, a one-time autologous TIL cell therapy, has demonstrated durable clinical benefit in this setting, with the C-144-01 study showing an ORR of 31.4% in a heavily pre-treated population. And the latest 4-year follow-up data from C-144-01 on lifileucel’s treatment outcomes and patterns of response were reported in 2023 ESMO_IO.
Lifileucel's R&D Progress
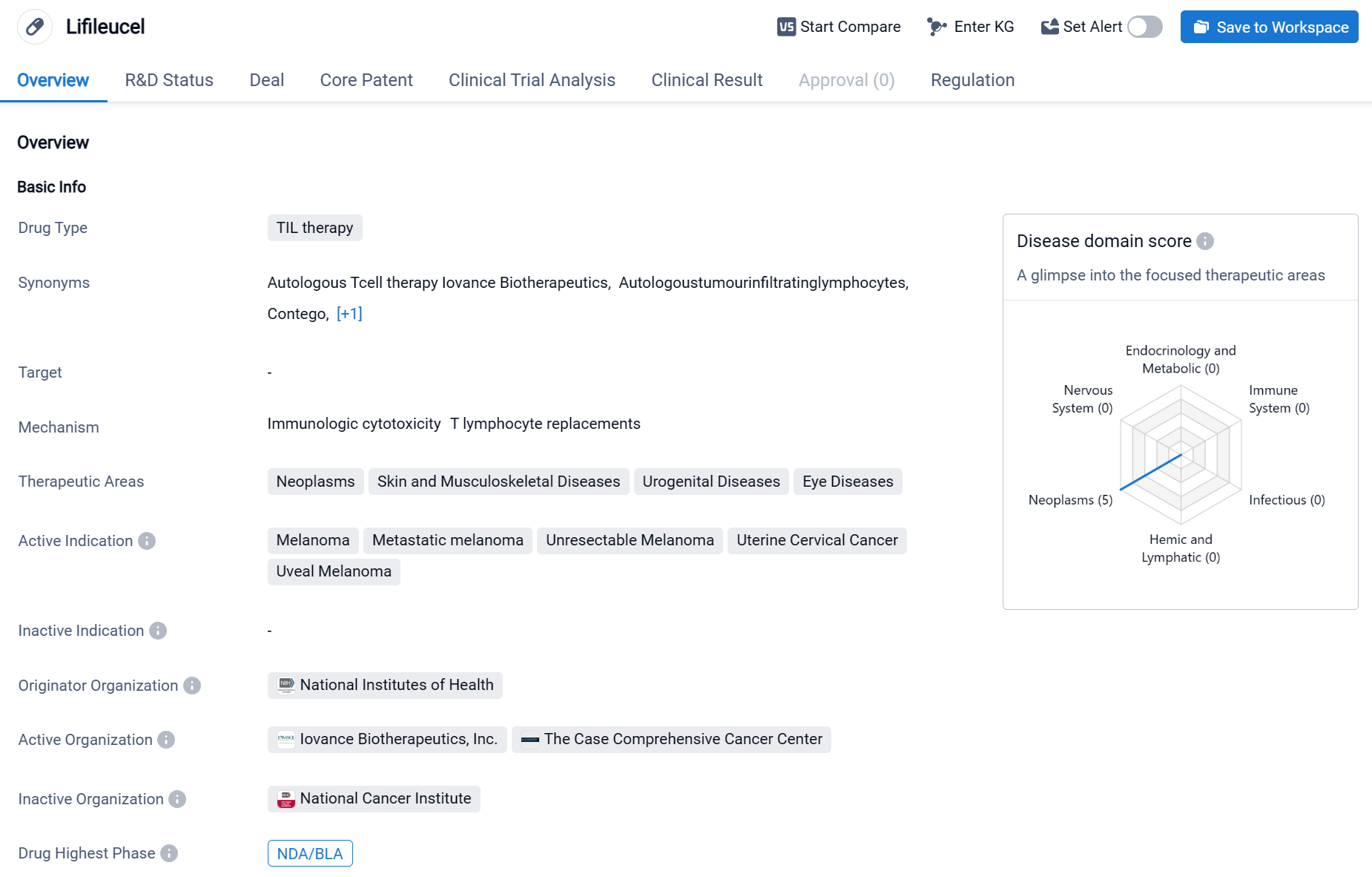
Lifileucel is a drug classified as TIL therapy, which stands for tumor-infiltrating lymphocyte therapy. It is primarily used in the treatment of various neoplasms, skin and musculoskeletal diseases, urogenital diseases, and eye diseases. The active indications for Lifileucel include melanoma, metastatic melanoma, unresectable melanoma, uterine cervical cancer, and uveal melanoma.
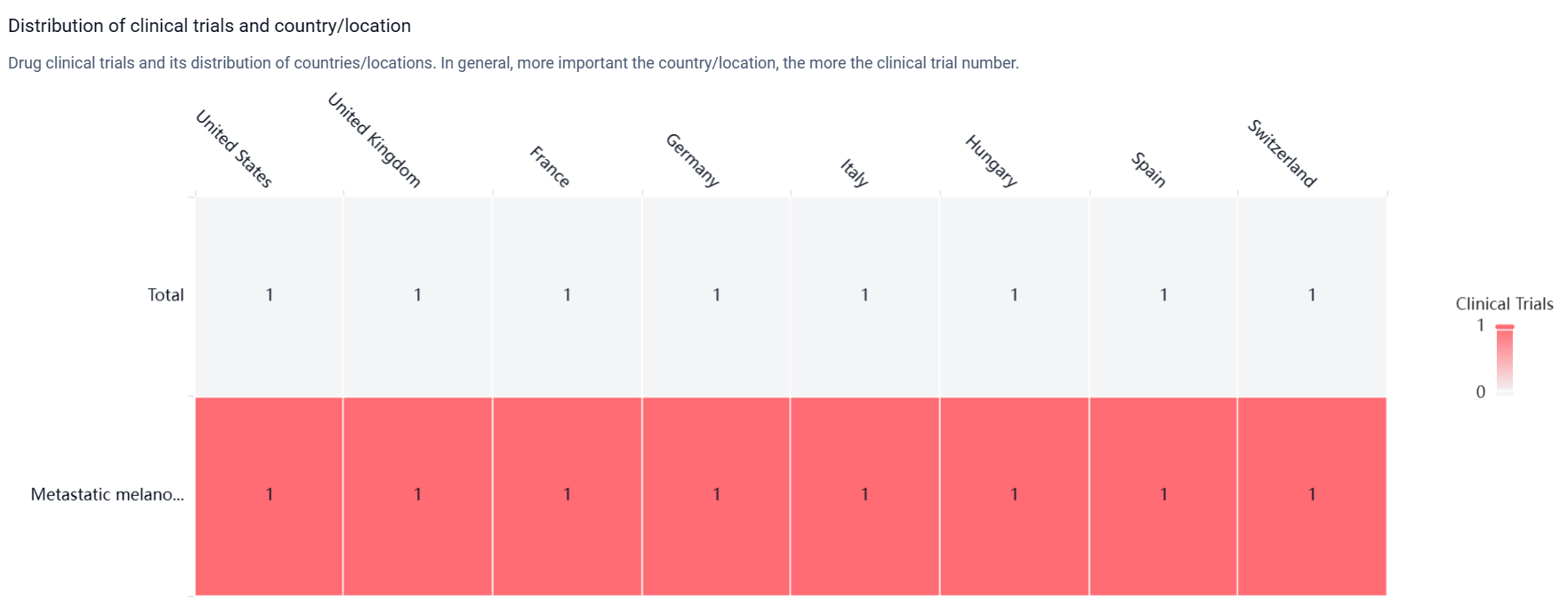
According to the Patsnap Synapse, Lifileucel was developed by the National Institutes of Health (NIH), a renowned organization in the field of biomedical research. Lifileucel has reached the highest phase of development, which is the New Drug Application (NDA) or Biologics License Application (BLA) stage. And the clinical trial distributions for Lifileucel are primarily in the United States, United Kingdom and France. The key indication is Metastatic melanoma.
Detailed Clinical Result of Lifileucel
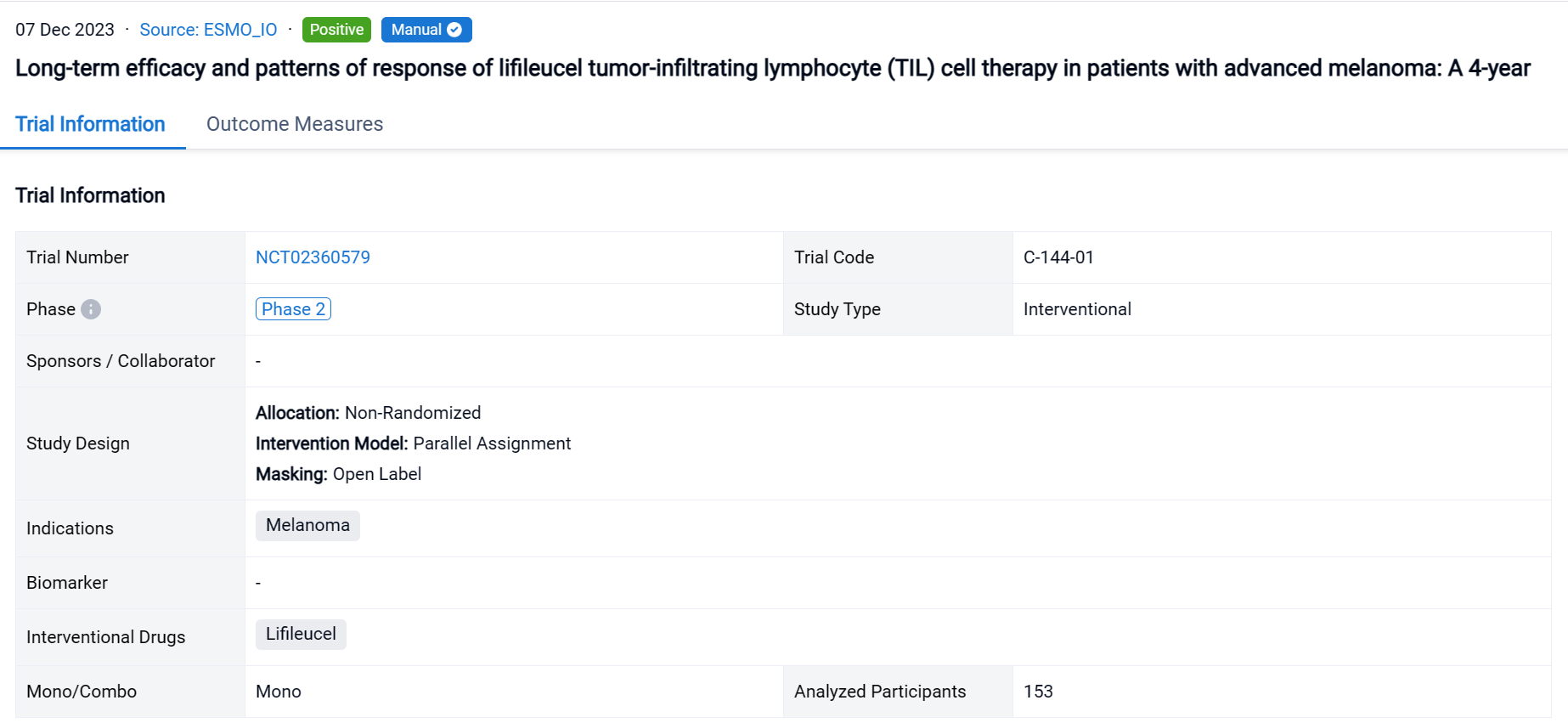
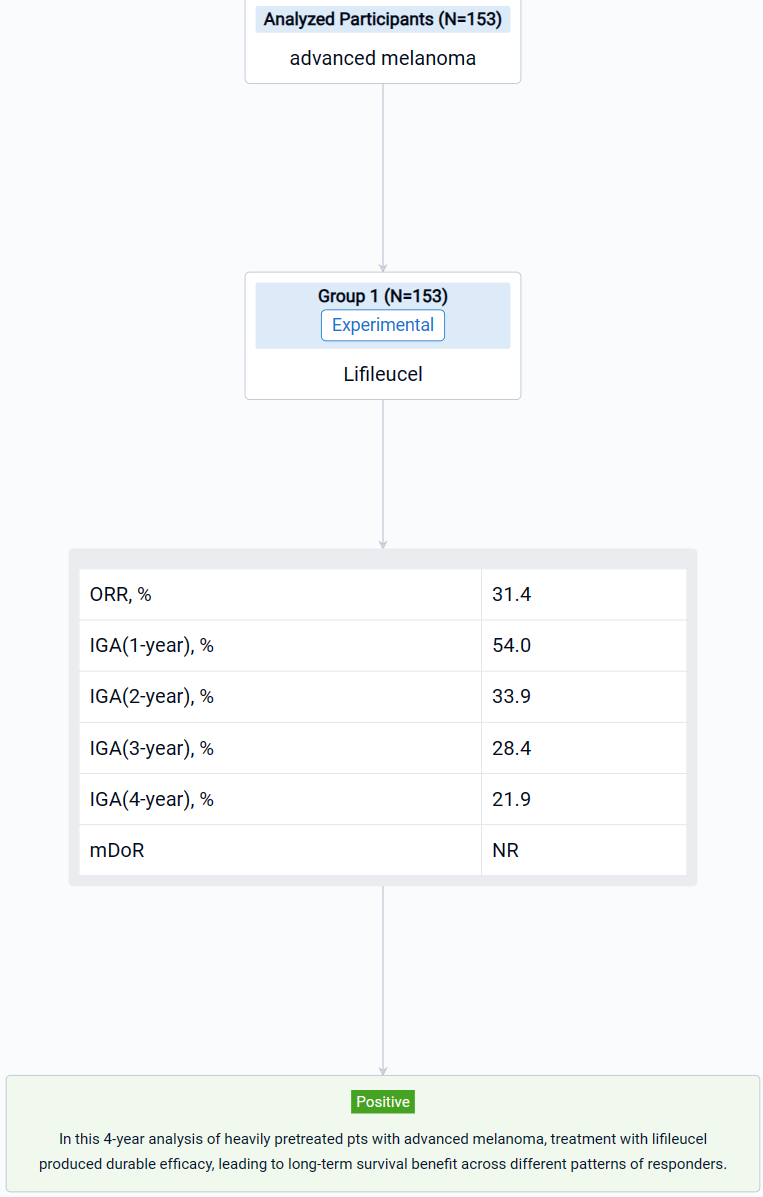
This non-randomized, parallel assignment, open-labeled clinical trial (NCT02360579) was aimed to explore the long-term efficacy and patterns of response of lifileucel tumor-infiltrating lymphocyte (TIL) cell therapy in patients with advanced melanoma.
In this study, pts had ≥1 lesion(s) resected (≥1.5 cm diameter) for 22-day cryopreserved lifileucel manufacturing. The treatment regimen consisted of nonmyeloablative lymphodepletion (cyclophosphamide 60 mg/kg/d × 2d, fludarabine 25 mg/m2/d × 5d), followed by a single lifileucel infusion and ≤6 doses of high-dose IL-2 (600,000 IU/kg).
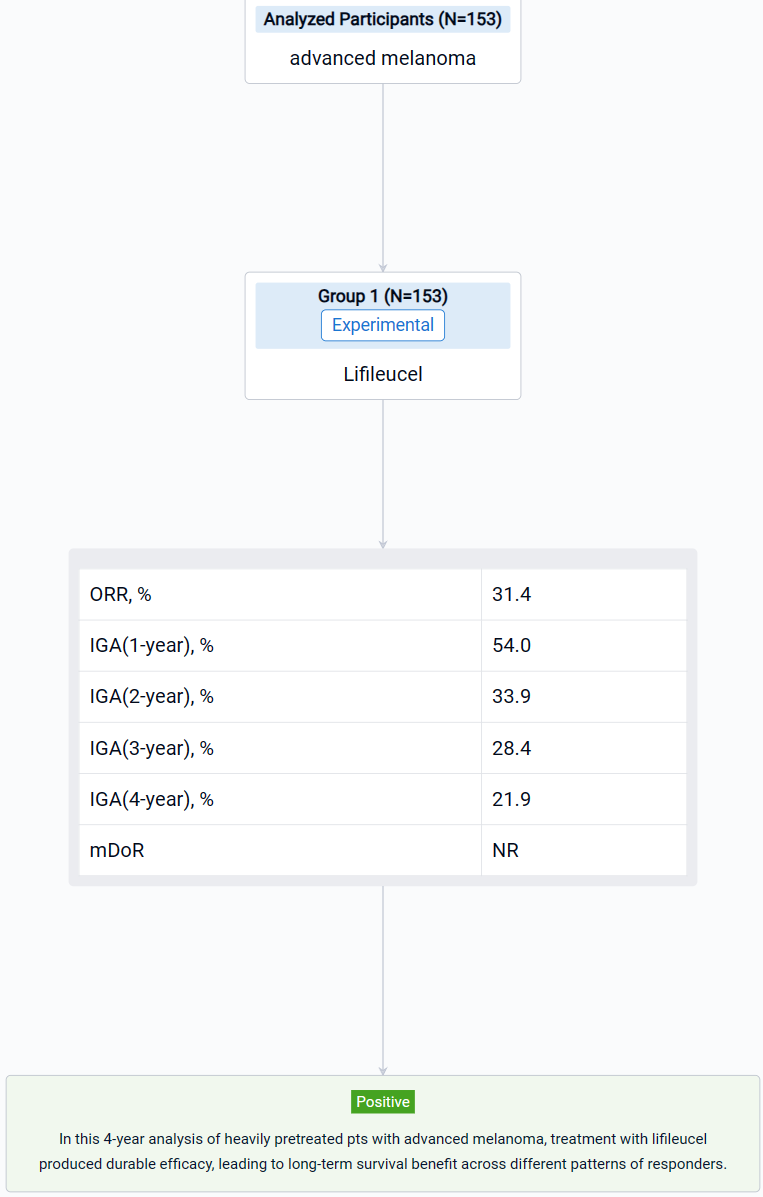
The result showed that as of data cutoff (30 June 2023), 153 pts were assessed; median study follow-up was 48.1 months. Independent review committee-assessed ORR by RECIST v1.1 was 31.4% with median DOR (months) of NR. The 1-, 2-, 3-, and 4-year OS rate was 54.0%, 33.9%, 28.4%, and 21.9%, respectively. Responders (n=48) had a median age of 55.0 years with median of 3 prior lines of therapies. Clinically meaningful 4-year OS rates were seen across all patterns of response (range, 37.2%–68.2%); pts with deepened response had numerically higher OS rate. Treatment-emergent adverse events were consistent with known safety profiles of lymphodepletion and IL-2.
It can be concluded that in this 4-year analysis of heavily pretreated pts with advanced melanoma, treatment with lifileucel produced durable efficacy, leading to long-term survival benefit across different patterns of responders.
How to Easily View the Clinical Results Using Synapse Database?
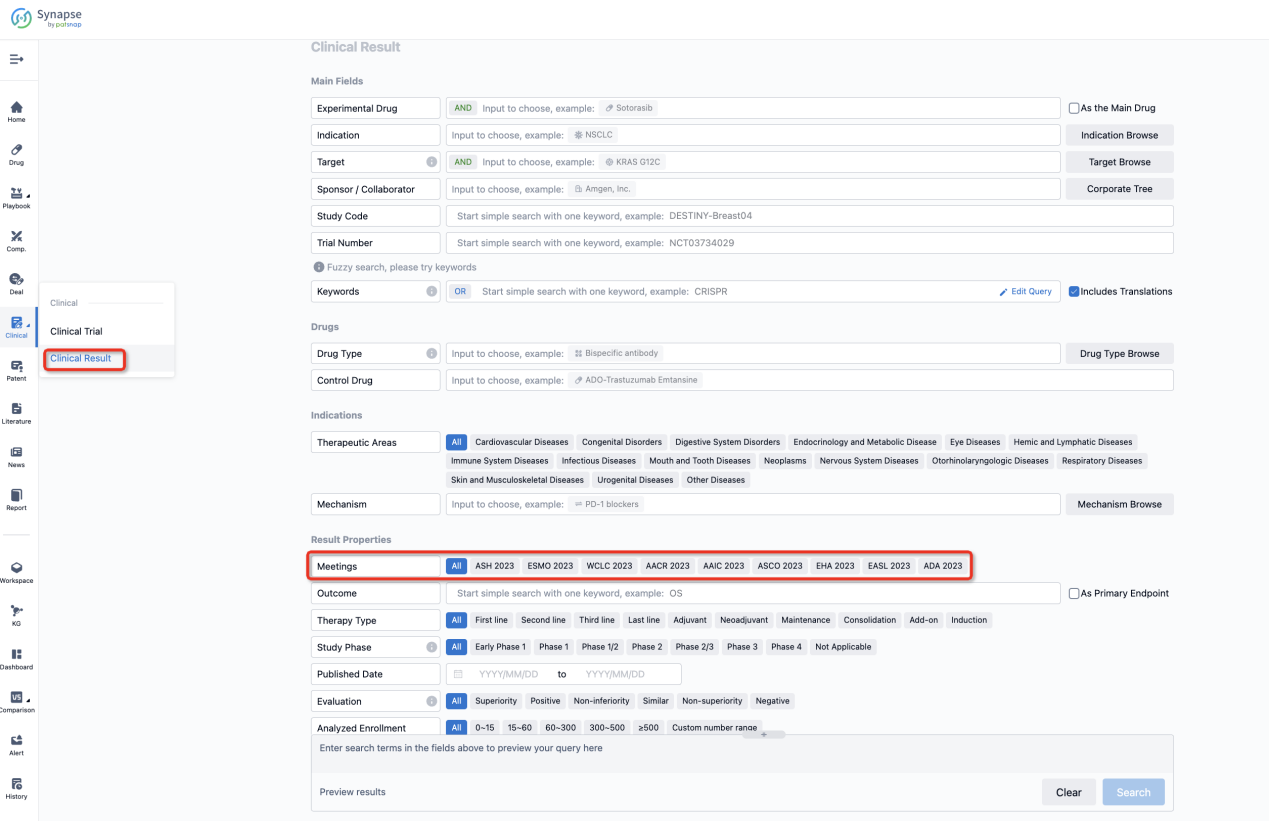
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
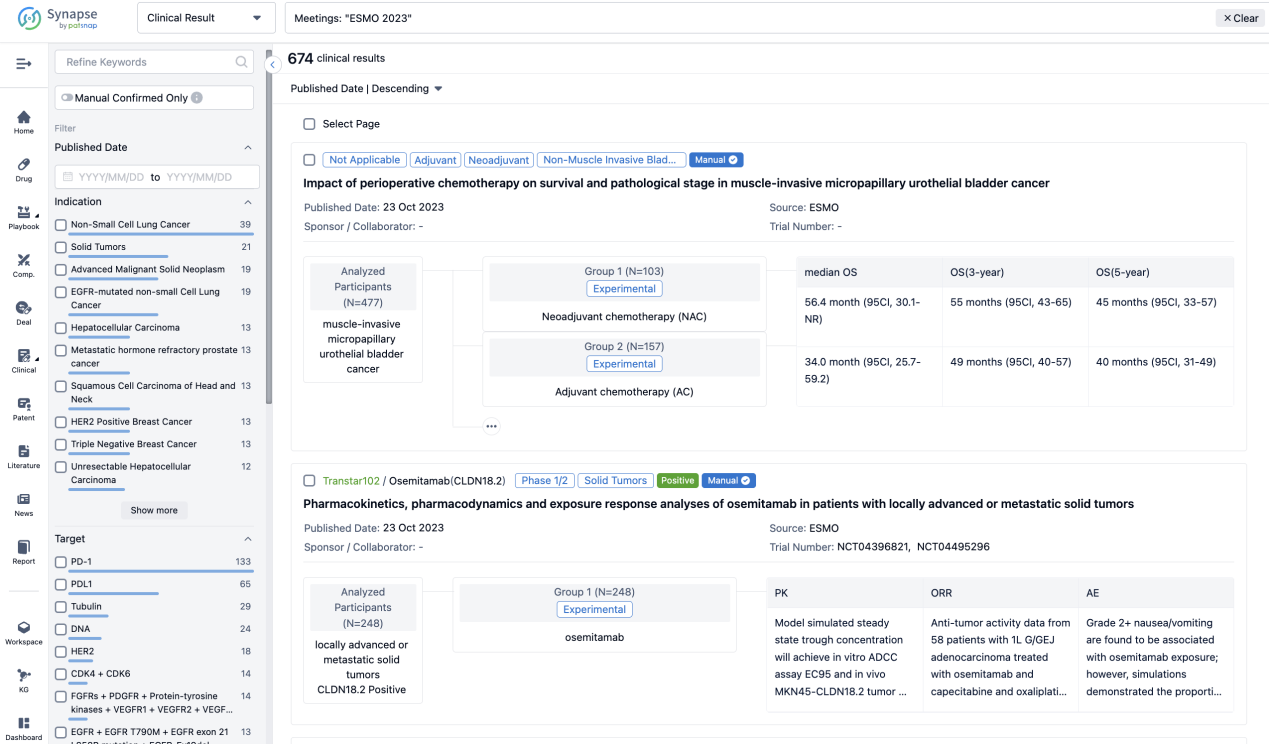
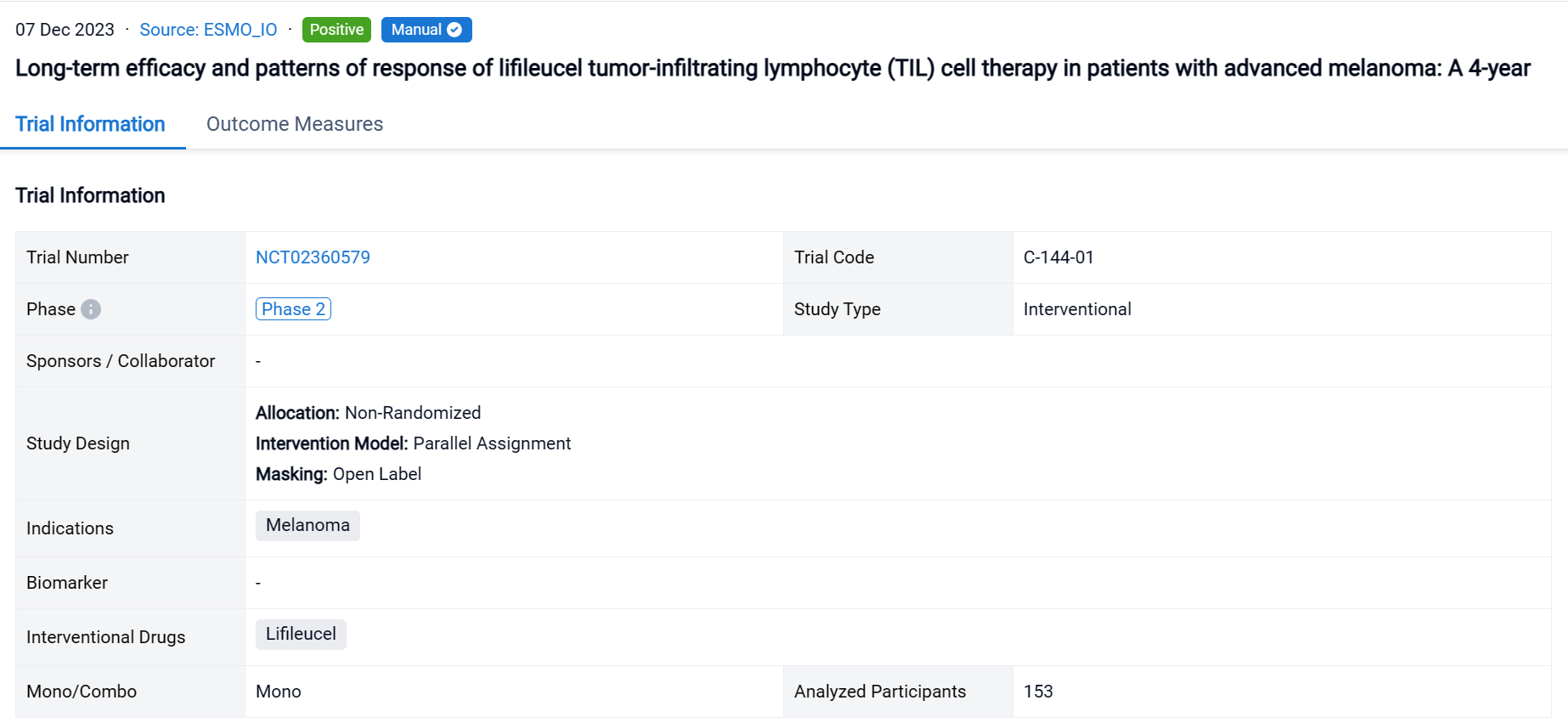
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
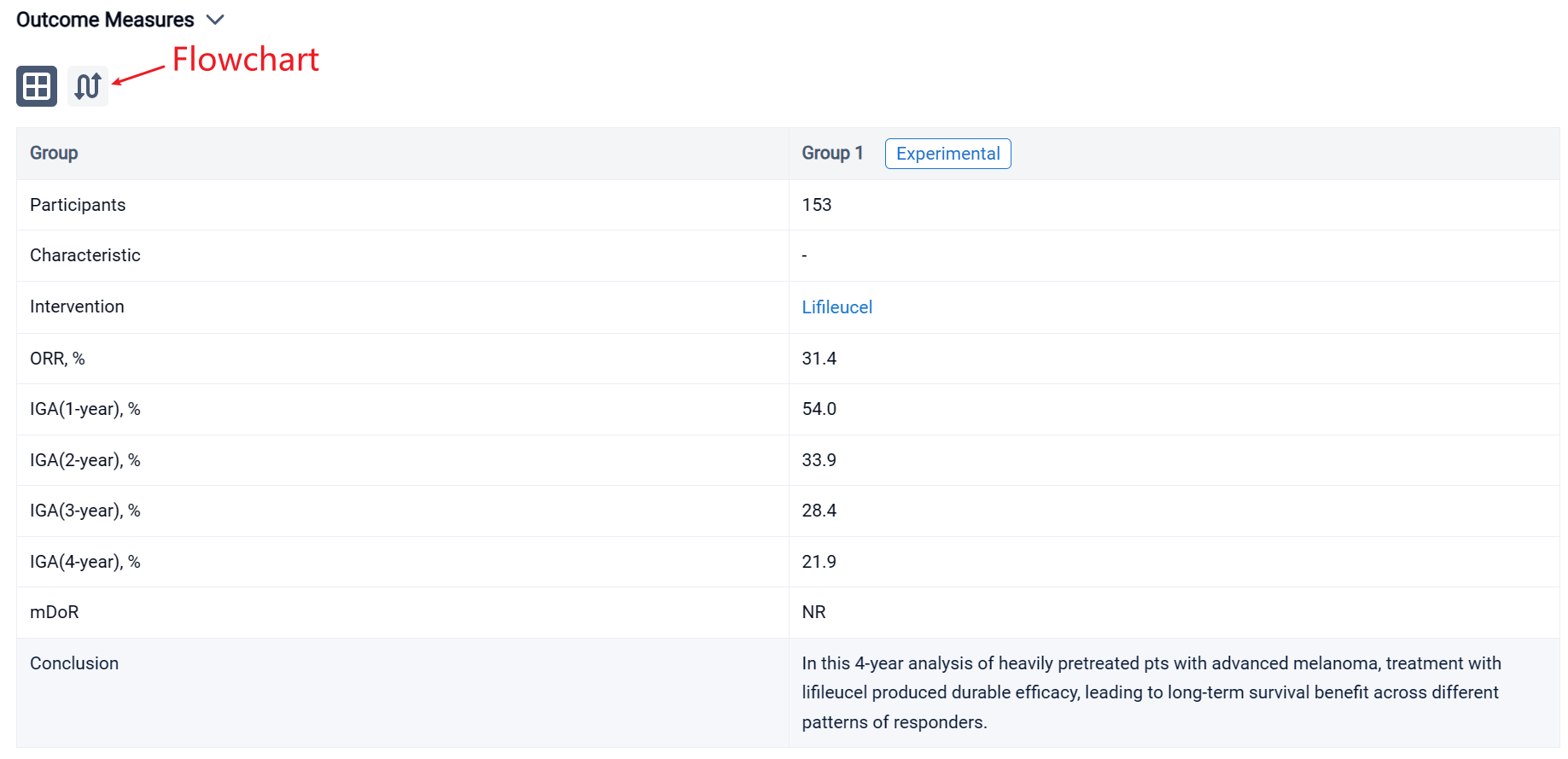
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
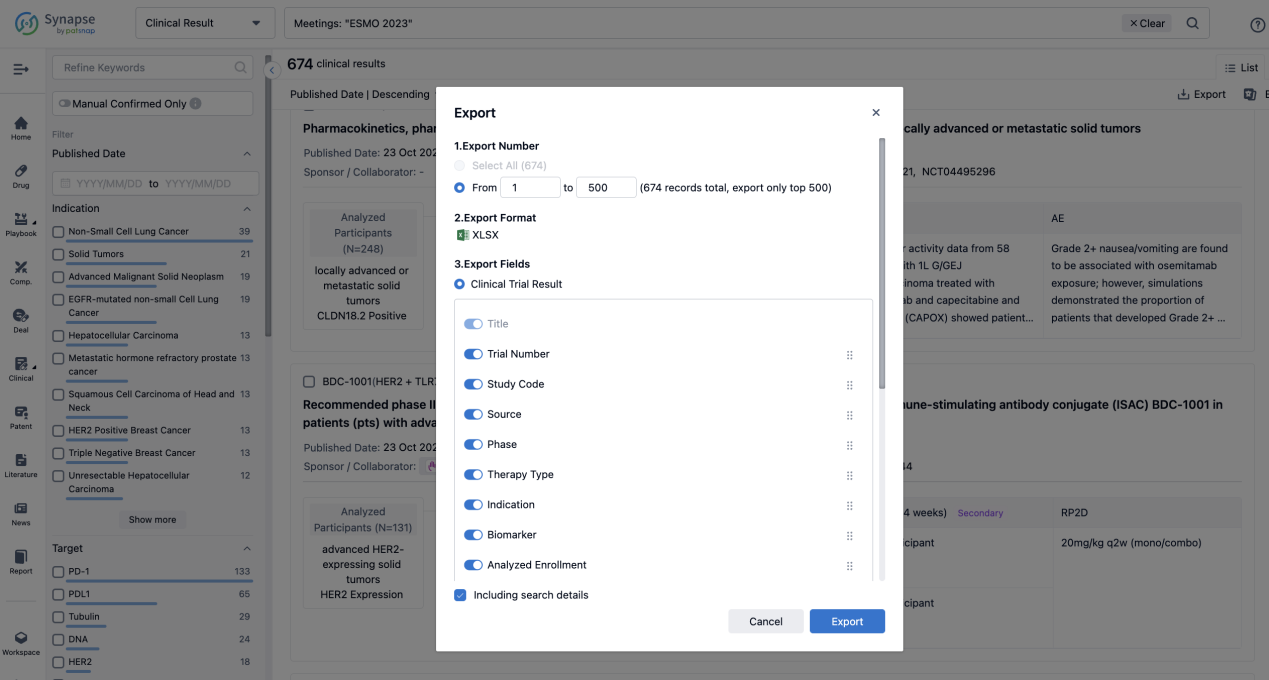
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!