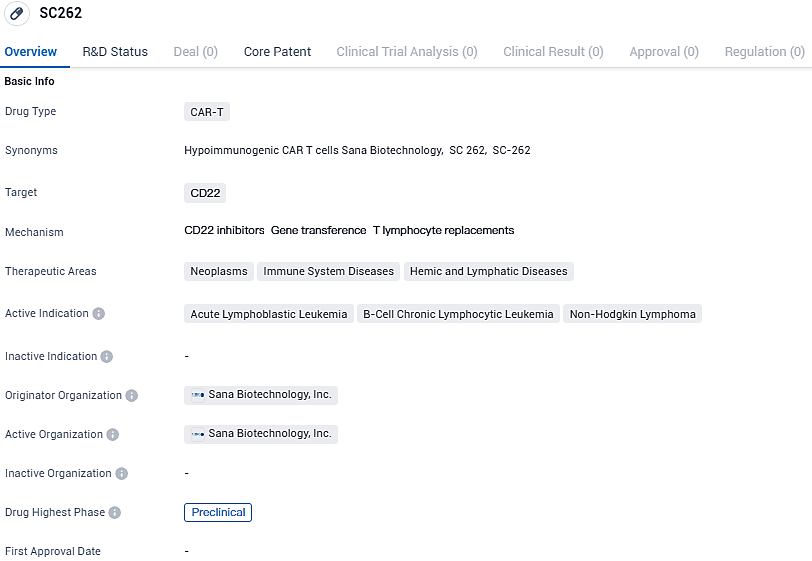
Sana Biotech's SC262, a CD22-targeted CAR T cell therapy for resistant B-cell cancers, gains US regulatory approval
Sana Biotechnology, Inc., an enterprise dedicated to transforming patient treatment possibilities via cell engineering, has revealed that the U.S. Food and Drug Administration granted approval for the firm to commence trials involving SC262 on individuals battling recurrent or treatment-resistant B-cell cancers. This investigation will primarily target those individuals who have previously undergone CAR T therapy targeting the CD19 antigen.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the treatment of B-cell cancers, specialized CAR T cell therapies have been developed with molecular constructs designed to recognize antigens present on B-cell surfaces. The antigen CD19 is a primary target for all existing individualized CAR T treatments approved for conditions such as B-cell lymphoma and B-cell acute lymphoblastic leukemia. Regrettably, around 60% of patients treated with CD19-specific CAR T cells experience partial remissions or disease recurrence. As a consequence, attention has shifted to CD22, another protein found on B cells, as a potential therapeutic alternative when CD19-focused approaches do not yield lasting complete remissions.
A novel therapeutic agent, SC262, possesses an identical CAR structure, inclusive of the same CD22-targeting component, as that utilized in various research-level clinical studies dedicated to CD22-specific CAR T therapy. To date, these studies have yielded long-term complete remissions in many patients who previously did not respond optimally to treatments targeting CD19.
"The rising number of patients who do not respond to CD19-targeting CAR T therapies indicates a pressing medical need," stated Doug Williams, PhD, Chief of Research and Development at Sana.
Dr. Williams elaborated, "In the recent year, Sana has secured three IND approvals from regulatory bodies and aided in the approval of a CTA for research, allowing the launch of new investigations in seven distinct areas related to cancer, B-cell driven autoimmune maladies, and type 1 diabetes, all under our hypoimmune technology umbrella. We are eager to share the findings from these studies within the year, including preliminary results for SC262 expected later in the cycle."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
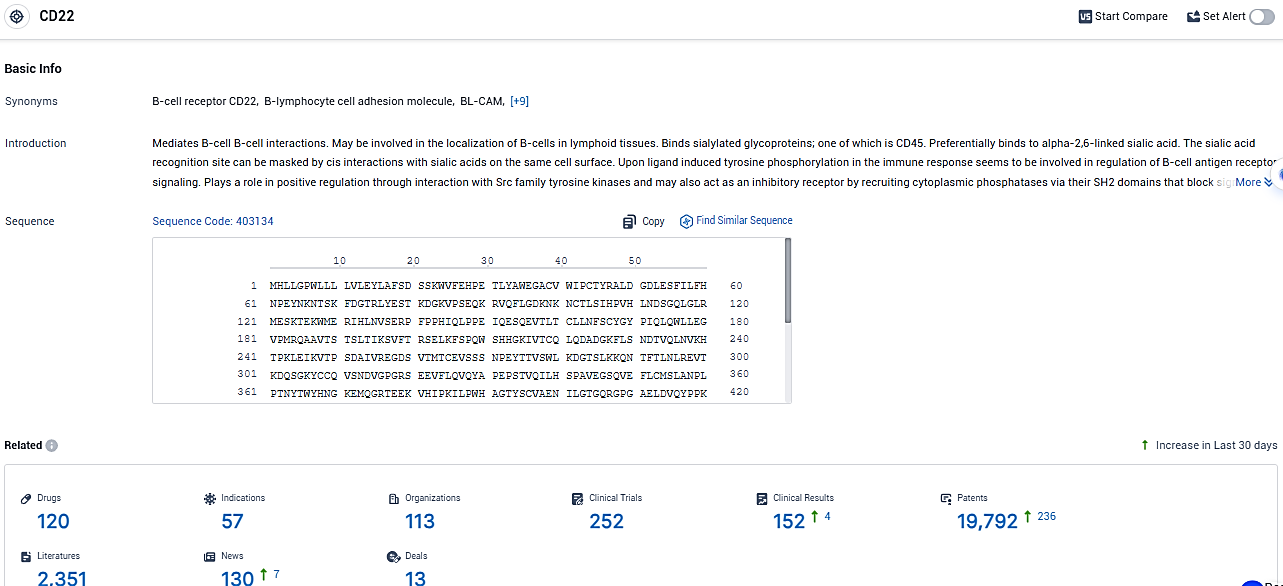
According to the data provided by the Synapse Database, As of January 12, 2024, there are 120 investigational drugs for the CD22 target, including 57 indications, 113 R&D institutions involved, with related clinical trials reaching 252, and as many as 19792 patents.
SC262 targets CD22 and is being investigated for its potential in treating acute lymphoblastic leukemia, B-cell chronic lymphocytic leukemia, and non-Hodgkin lymphoma. Currently in the preclinical phase, SC262 is still undergoing testing to determine its safety and efficacy.