Boston Pharma Debuts BOS-580 Study at 2024 NASH-TAG, Highlights Lasting Low Immune Response for MASH Treatment
Boston Pharmaceuticals, an enterprise operating in the clinical-phase biopharmaceutical sector, focused on crafting unique molecular compounds aimed at tackling critical hepatic ailments, has disclosed supplementary results from its Phase 2 studies. These new findings bolster the evidence for BOS-580's minimal likelihood of triggering immune responses, in addition to its promising therapeutic impact.
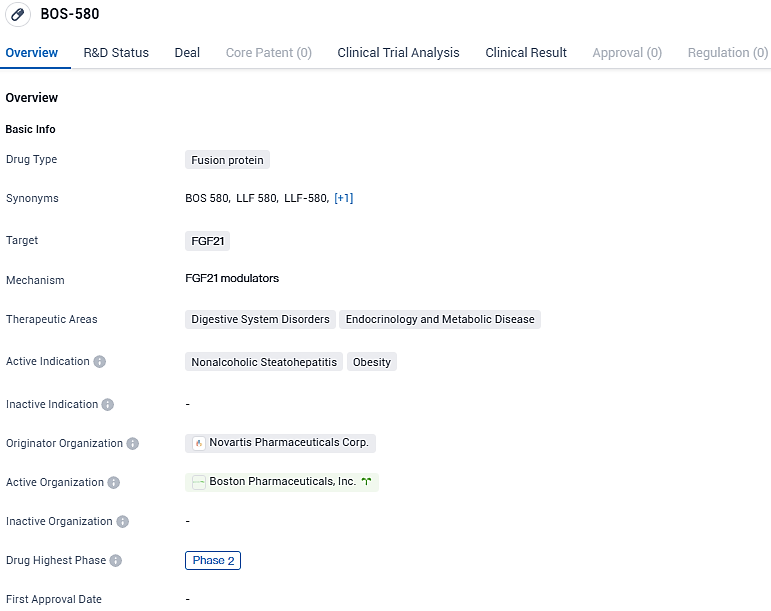
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
BOS-580, a sustained-release variant of the fibroblast growth factor 21 (FGF21) analogue, is formulated specifically for addressing the complications tied to metabolic dysfunction-associated fatty liver disease, commonly known as non-alcoholic steatohepatitis (NASH). Revelations about this treatment were shared at the prestigious NASH-TAG conference of 2024, held in Park City, Utah.
Speaking on the recent findings, Boston Pharmaceuticals' CEO Sophie Kornowski remarked, "The data we've gathered offers promising insights, suggesting that BOS-580 may not evoke a significant immune response leading to the production of antibodies that counteract the drug. This remains an essential concern for those focusing on developing analogues of FGF21 for managing MASH, since prolonged and consistent efficacy is crucial for patient care." Kornowski highlighted the feature that sets BOS-580 apart from similar drugs under development – its potential to confer a low likelihood of immunogenic reactions.
Furthermore, a separate analysis deriving from Boston Pharmaceuticals' Phase 2a clinical research illustrated the marked influence BOS-580 has on a variety of non-invasive biomarkers indicative of liver inflammation and scarring, specifically in patients with MASH.
"Our current findings underscore the promise held by BOS-580 in becoming a central element of therapeutic regimens for individuals grappling with MASH," shared Juan Carlos Lopez-Talavera, M.D., Ph.D., CMO of Boston Pharmaceuticals. "We are moving with purpose towards introducing a once-monthly BOS-580 administration to critical phase clinical studies in the immediate future. Our aspiration is that BOS-580 will emerge as an effective and convenient therapeutic solution for those afflicted by MASH."
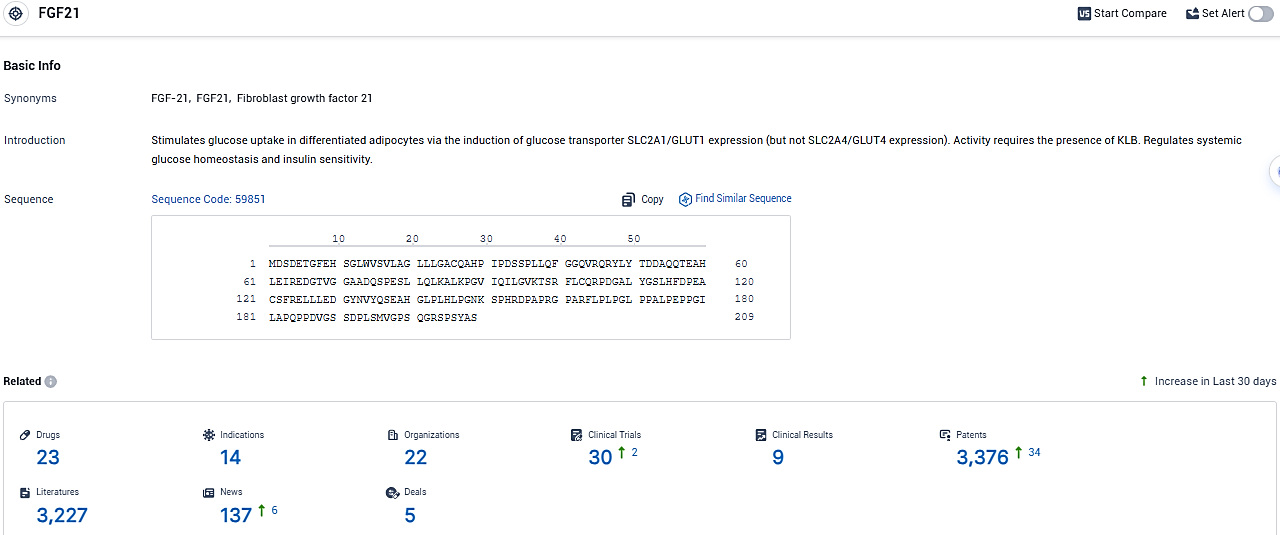
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 10, 2024, there are 23 investigational drugs for the FGF21 target, including 14 indications, 22 R&D institutions involved, with related clinical trials reaching 30, and as many as 3376 patents.
BOS-580 is a once-monthly subcutaneous injectable of a long-acting, highly engineered variant of human fibroblast growth factor 21 that regulates various metabolic pathways to decrease liver fat and ameliorate liver inflammation and damage in patients with metabolic dysfunction-associated steatohepatitis, also known as non-alcoholic steatohepatitis. Currently in Phase 2 of clinical development, BOS-580 shows potential in addressing the therapeutic needs in the field of digestive system disorders and endocrinology and metabolic diseases.