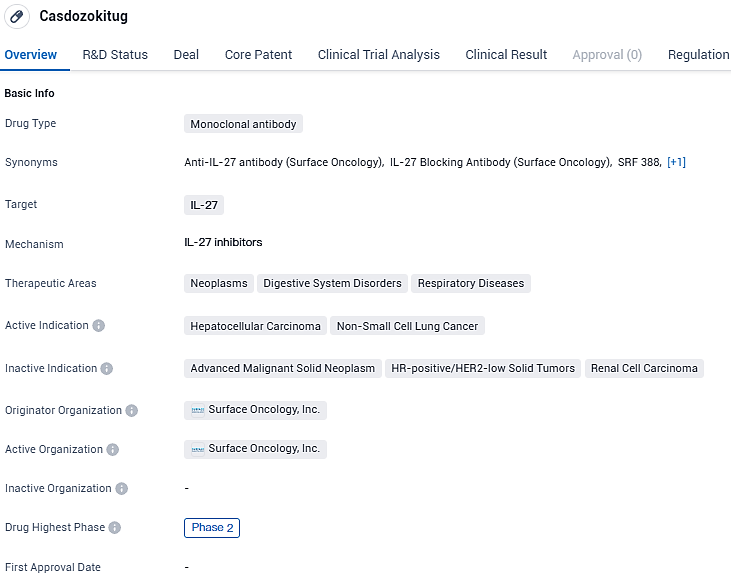
At the 2024 ASCO GI Symposium, Coherus presented promising Phase 2 results for the novel IL-27-targeted antibody, Casdozokitug
Coherus BioSciences, Inc.,, released details from the initial segment of the Phase 2 clinical study. In this study, they assessed a highly selective and powerful IL-27-targeted antibody, casdozokitug (casdozo), used alongside atezolizumab and bevacizumab. The trial involved patients with locally advanced or metastatic hepatocellular carcinoma, who hadn't previously received any treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The 2024 ASCO Gastrointestinal Cancers Symposium will showcase these findings from January 18-20, 2024 at Moscone West in San Francisco, California. Interleukin (IL)-27 is a crucial regulatory cytokine suppressing anti-tumor immune responses and has emerged as a pivotal target for cancer therapies. Casdozo, a pioneering antibody and the only clinical phase cytokine antagonist that challenges IL-27, is a significant development.
For liver cancer patients, for whom surgery isn't a viable option or those whose cancer has metastasized, dual immunotherapy combinations have shown improved outcomes. Nevertheless, there's a gap in response to the current therapies amongst patients, leading to a demand for innovative treatment methods that can enhance survival rates and not pose extra toxicity,” noted Daneng Li, M.D., and Associate Professor in the Department of Medical Oncology & Therapeutics Research and Co-Director of City of Hope Comprehensive Cancer Center.
He further added, “The integration of casdozo to the standard care is promising and supports further scrutiny of cazdozo, and its innovative anti-IL-27 mechanism, as part of tri-combination therapy in HCC. Moreover, each person suffering from advanced HCC's circumstances are unique, factoring in their tumour, comorbidities and other variables – this makes the biomarker data showing a relationship between IL-27 biology and the response to casdozo particularly intriguing. Possibility to discern biomarkers of response could potentially bring immense benefits to patients with liver cancer.”
Rosh Dias, M.D., Coherus’ Chief Medical Officer, stated, “Our data now covers several tumor types for casdozo reflecting clinical activity. Our holistic clinical development program includes current and future clinical trials featuring casdozokitug in conjunction with our anti-PD-1 antibody foundation of toripalimab-tpzi.
Our goal is to further refine our in-house, next-gen immuno-oncology combinations that aim to conquer immune suppression within tumour microenvironments, thereby increasing longevity and bettering patient outcomes.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
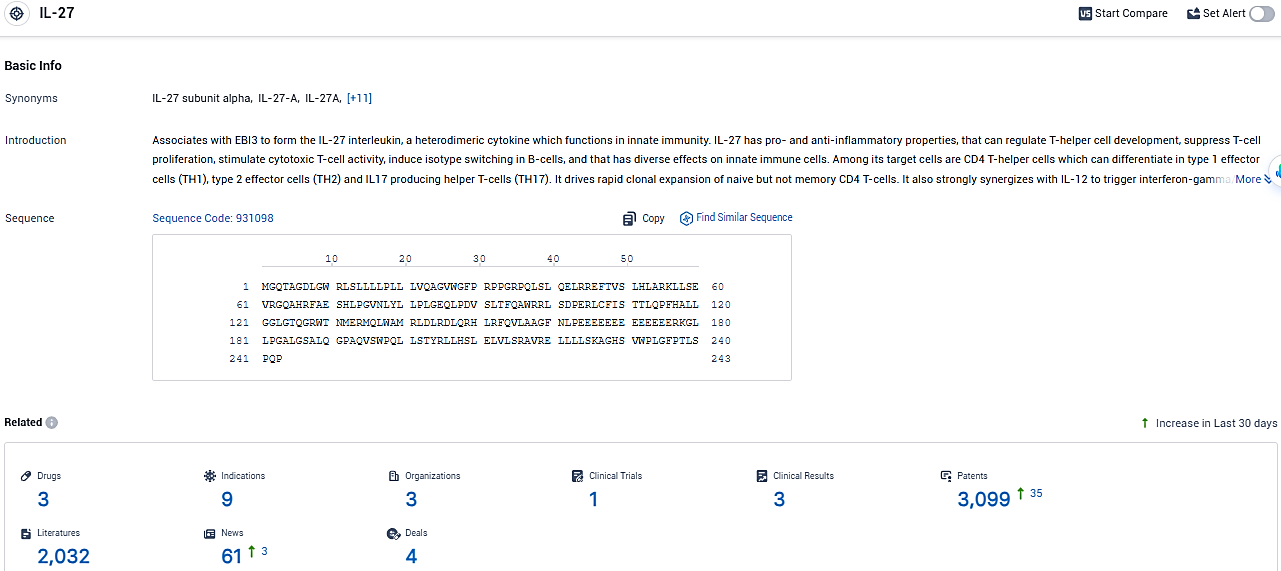
According to the data provided by the Synapse Database, As of January 25, 2024, there are 3 investigational drugs for the IL-27 target, including 9 indications, 3 R&D institutions involved, with related clinical trials reaching 1, and as many as 3099 patents.
Casdozokitug is a first-in-class human anti-IL-27 antibody designed to inhibit the activity of this immunosuppressive cytokine. Casdozokitug has been granted Orphan Drug designation and Fast Track designation for the treatment of refractory hepatocellular carcinoma from the FDA. It is the first IL-27 antibody to enter the clinic.