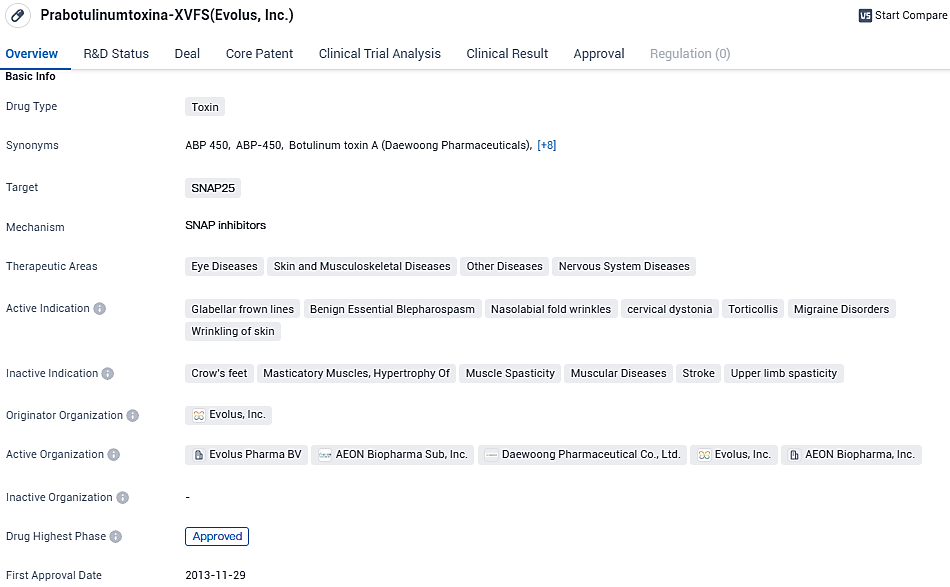
AEON Biopharma Reports Promising ABP-450 Results for Cervical Dystonia and PTSD at Neurotoxin Conference
At the International Neurotoxin Association's premier conference, TOXINS 2024, held at the Estrel Berlin in Berlin, Germany from January 17th through 20th, AEON Biopharma, Inc., offered fresh pre-clinical and clinical research data for ABP-450. This company, which is currently at the clinical stage, concentrates on creating an exclusive botulinum toxin complex to manage various serious medical conditions, including cervical dystonia and posttraumatic stress disorder.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Effectiveness and Safe Use of ABP-450 (prabotulinumtoxinA) in Adult Cervical Dystonia Patients: Findings from the Continuation of an Open-label Phase 2 Experiment," was reported by Chad Oh, M.D., AEON's Senior Medical Officer. Preliminary findings from the recently concluded extension of the Phase 2 experiment reveal that ABP-450 was generally safe and well received in CD patients during the study, up to 4 treatment cycles in a one-year period.
In the study, there was no evidence that ABP-450 increased instances of treatment-related TEAEs at any dosage. Optimal effectiveness for all dosages and cycles of ABP-450 was seen as early as the first month, with the effect remaining from 12 to 16 weeks post-treatment. These outcomes provide encouragement for the further examination of ABP-450 as a potential remedy for CD, along with other movement disorders.
"Highly promising results from the 52-week OLE part of the ABP-450 Phase 2 experiment for CD has us eager to share the positive news," said AEON's Chief Executive Officer and President, Marc Forth. "Based on these findings, we think further scrutiny on the use of ABP-450 in managing CD as well as other movement disorders is warranted, and we anticipate taking our CD project for ABP-450 forward to a potential Phase 3 trial." "We eagerly await a discussion with FDA concerning the CD data collected so far, which includes findings from the OLE, to finalize the design of a Phase 3 experiment for the use of ABP-450 in managing CD."
ABP-450 is made up of a 900 kDa botulinum toxin type-A complex that is produced by the bacterium Clostridium botulinum. At therapeutic dosages, ABP-450 inhibits the release of peripheral acetylcholine at presynaptic cholinergic nerve terminals by breaking down SNAP-25, a key protein in the successful docking and release of acetylcholine from vesicles situated within nerve endings, leading to muscle relaxation and denervation.
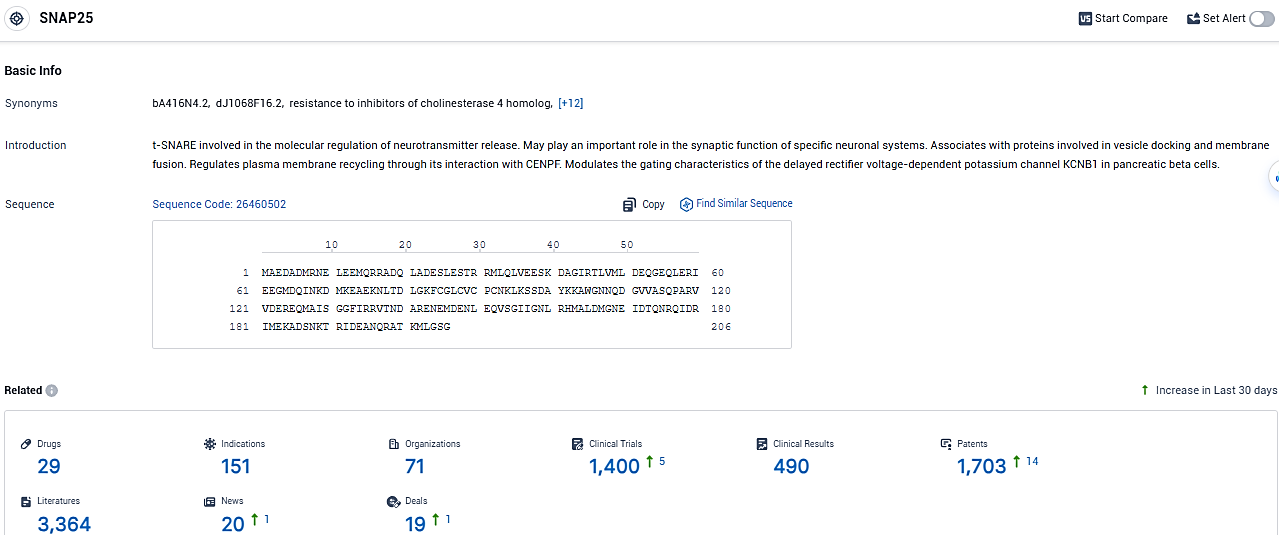
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 25, 2024, there are 29 investigational drugs for the SNAP25 target, including 151 indications, 71 R&D institutions involved, with related clinical trials reaching 1400, and as many as 1703 patents.
ABP-450 shows promise as a versatile drug with potential applications in various therapeutic areas. Its approval in South Korea and ongoing regulatory processes in China indicate its progress towards becoming available to a wider patient population. As an expert in the pharmaceutical industry, it would be important to closely monitor the developments and potential market opportunities for ABP-450.