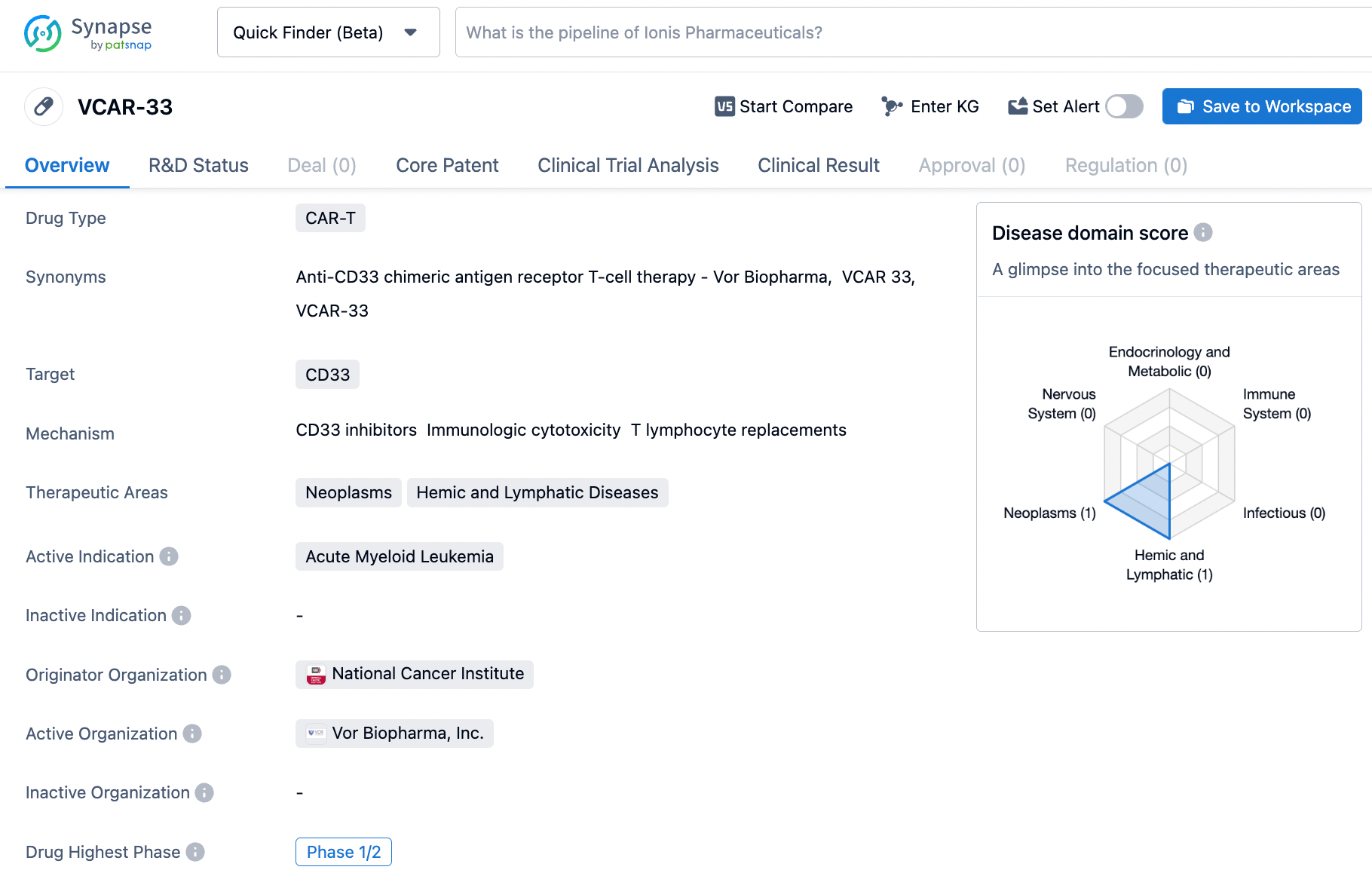
Vor Bio Begins Treating First Patient with Acute Myeloid Leukemia Using VCAR33 (ALLO) and Provides Company Update
Vor Bio, an enterprise focused on cell and genome alteration currently in the clinical phase, disclosed that they began treatment on the first enrollee in VBP301, a first-in-human, multicenter, non-blinded Phase 1/2 trial of VCAR33ALLO today. The company managed to prolong its monetary sustainability to the latter half of 2025.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Kicking off 2024 with robust performance is gratifying, and we eagerly anticipate delivering preliminary clinical outcomes from the VBP301 trial in the coming year," declared Dr. Eyal Attar, Chief Medical Officer of Vor Bio. "We are thrilled to offer AML patients a groundbreaking CAR-T developmental therapy formulated from a healthy transplant donor, which could counterbalance the deficiencies observed in either autologous or allogeneic off-the-shelf methodologies for the first time."
Earlier, a patient suffering from refractory / relapsed acute myeloid leukemia received a dose of VCAR33ALLO during the VBP301 clinical trial. This event marked an important achievement indicating the successful manufacturing of VCAR33ALLO in Vor Bio's in-house facility.
VCAR33ALLO is prepared from a patient’s original transplant donor lymphocytes resulting in a CAR-T cellular product that perfectly corresponds to the recipient's engrafted blood system. The technique of using lymphocytes from a healthy transplant donor to develop VCAR33ALLO gives the CAR-T cells a stem-like characteristic, increasing their capacity to expand, persist, and display anti-leukemia activity compared to that derived from the patient's lymphocytes.
Notably, the opportunity to treat patients with relapsed trem-cel transplants using VCAR33ALLO under the VBP301 protocol could provide early potential insights into the company’s trem-cel + VCAR33 Treatment System, which uses VCAR33 after a trem-cel transplant to diminish the chances of relapse or treat the signs of relapse. The first case was a patient who had a recurrence following a standard care transplant, though patients who have recurred after a trem-cel transplant are also qualified to enroll. The preliminary data from the VBP301 trial is projected to be available in the latter half of 2024.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
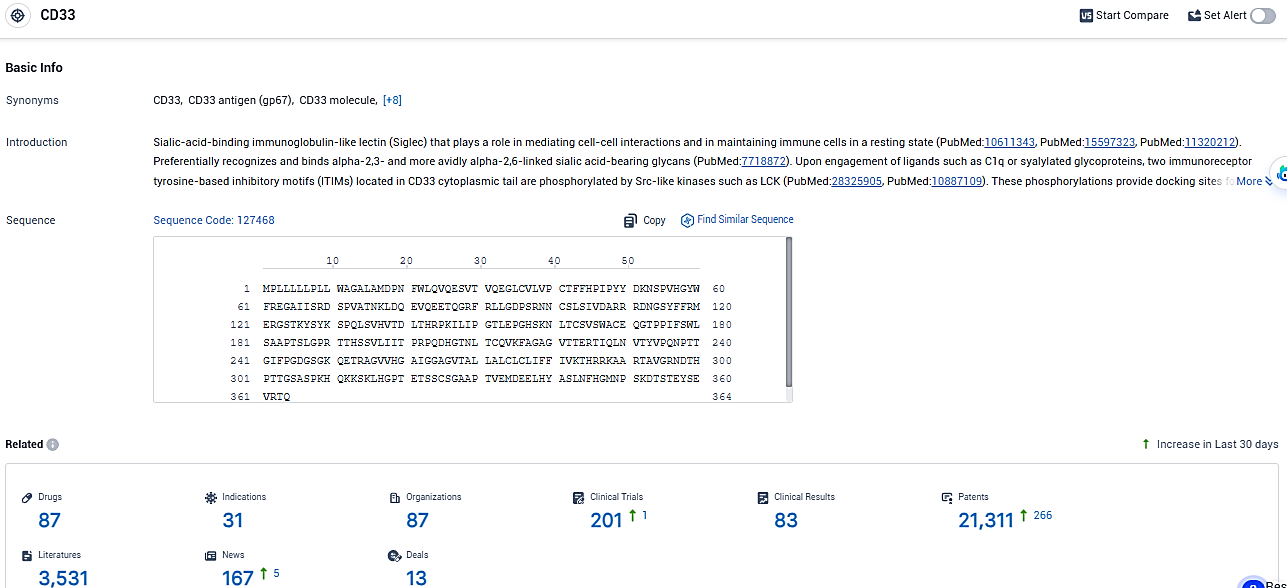 According to the data provided by the Synapse Database, As of January 24, 2024, there are 87 investigational drugs for the CD33 target, including 31 indications, 87 R&D institutions involved, with related clinical trials reaching 201, and as many as 21311 patents.
According to the data provided by the Synapse Database, As of January 24, 2024, there are 87 investigational drugs for the CD33 target, including 31 indications, 87 R&D institutions involved, with related clinical trials reaching 201, and as many as 21311 patents.
VCAR33ALLO is a CD33-directed CAR-T cell therapy made from healthy cells obtained from the same donor from which the patient was previously transplanted. VCAR-33 shows promise as a potential treatment for AML. However, it is important to note that the drug is still in the early stages of development, and further clinical trials will be necessary to determine its safety and efficacy. If successful, VCAR-33 could provide a new therapeutic option for patients with AML, addressing an unmet medical need in the field of biomedicine.




