Evommune Announces Early Trial for New MRGPRX2 Inhibitor to Treat Chronic Hives
Evommune, Inc., a firm in the clinical phase of biotechnology specializing in discovering and advancing novel methods to counter immune-mediated inflammatory diseases, has disclosed the commencement of its Phase 1 first-of-its-kind study. The study is designed to assess EVO756 in both healthy adults and those suffering from chronic spontaneous urticaria.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
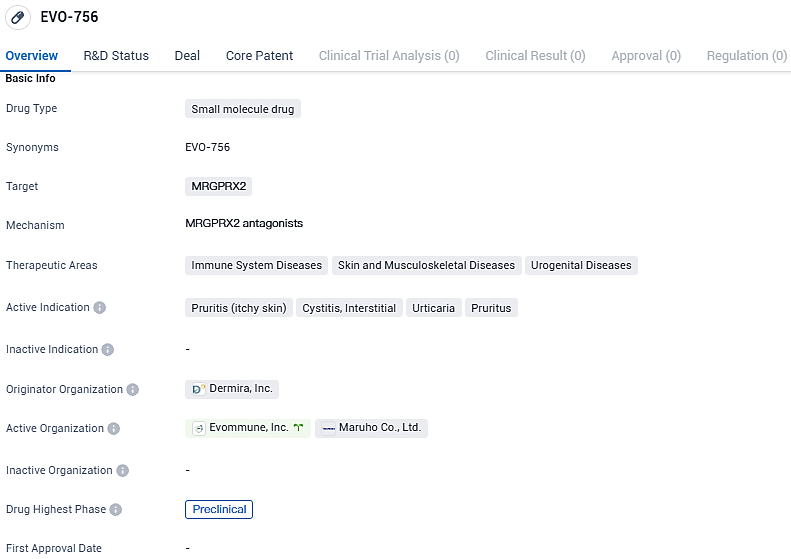
EVO756's ability to inhibit MRGPRX2 triggering and mast cell degranulation proposes that it could serve as a premier oral therapy for several diseases induced by mast cells, encompassing chronic inexplicable urticaria and provoking itching.
"We are thrilled to commence the initial dosage of EVO756 for the first patient in our preliminary Phase 1 experimentation,” expressed Eugene Bauer, M.D., Evommune's Principal Medical Officer.
"Our focus is on mast cells via the precise regulation of MRGPRX2, intending to produce an innovative treatment that possesses both the effectiveness of a biologic and the potential for oral administration on a daily basis, circumventing the safety risks that other mast cell extinguishing possibilities pose. In patients with CSU, an embargo on the MRGPRX2 receptor and its subsequent effects holds the ability to address the core cause of inflammation, providing improved relief over currently available treatments," Bauer elaborated.
The first phase of the experiment is a randomized, blind, placebo-controlled, exploration comprising both single and multiple ascending dosages in healthful adults and a publicly disclosed study in CSU-afflicted adults. The study is intended to gauge the safety parameters, tolerability, as well as the pharmacokinetics, of administering EVO756 orally.
This segment of the study will be executed in association with Sarbjit Saini, M.D., located at Johns Hopkins University. This allows the assessment of patients within a strictly regulated environment and has the advantage of simulating the effect of EVO756 compared with a standard against provoked urticarias within the cohort of healthy adults.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
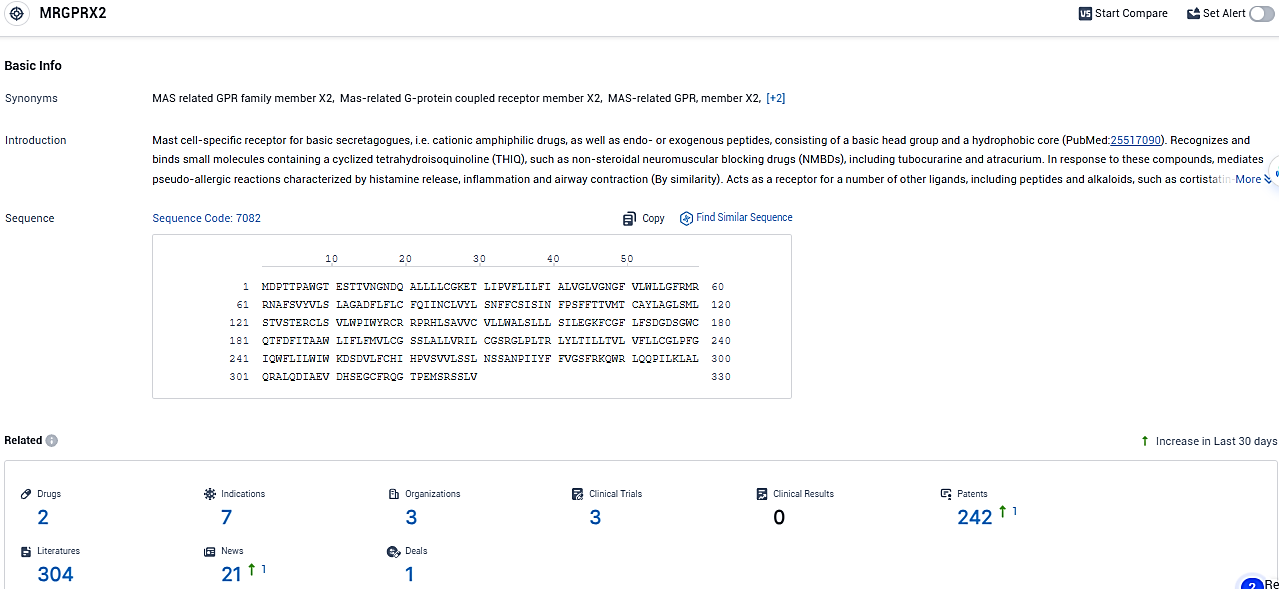
According to the data provided by the Synapse Database, As of January 24, 2024, there are 2 investigational drugs for the MRGPRX2 target, including 7 indications, 3 R&D institutions involved, with related clinical trials reaching 3, and as many as 242 patents.
EVO756 is a potent, highly selective small molecule antagonist of mas-related G-protein coupled receptor X2. EVO756 represents a new, targeted approach to the treatment of these disorders with the potential for once-daily oral administration without the serious side effects observed with other approaches.