Atai Strategic Investment in Beckley Psytech to Jointly Develop Rapid-Acting Psychedelic Medications for the Treatment of Depression
In January, the clinical-stage biopharmaceutical company atai Life Sciences (NASDAQ: ATAI), which is dedicated to revolutionizing the treatment of mental illnesses, announced a strategic investment in Beckley Psytech, a partnership that solidified atai's status as the bio-pharma company with the largest and most diversified portfolio of clinical-stage psychedelic drug candidates.

Beckley Psytech is a private clinical-stage biotechnology company focused on transforming short-acting psychedelics into highly efficacious, rapid-acting therapeutics for neuropsychiatric diseases. This strategic investment and collaboration will integrate Beckley Psytech's two clinical-stage short-acting psychedelic candidates, BPL-003 and ELE-101, into atai's mental health innovation platform, accelerating the development of these two drugs.
Per the terms of the investment, atai will hold a 35.5% stake in Beckley Psytech, which will remain an independent private company. The total investment amounts to $50 million, with $40 million directly invested in the company to fund ongoing research projects, and an additional $10 million used to purchase secondary shares from existing shareholders. atai will also have the right to appoint and hold three of the nine board seats at Beckley Psytech, and will have a time-limited right of first refusal on future company sales, asset sales or transfers of other commercial rights, as well as indefinite rights of first negotiation for BPL-003 and ELE-101.
The active ingredient of BPL-003 is a novel short-action intranasal formulation of 5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT, also known as Mebufotenin), which is found in various plant species and is also secreted by the glands of the Colorado River toad. Structurally similar compounds, DMT and bufotenin (5-HO-DMT), are also used as psychedelics in South America.
Early studies have shown that 5-MeO-DMT has the highest binding affinity to the 5-HT1A receptor (1.9-3 nM), with a selectivity ratio of 300-1000 times higher than that of the 5-HT2A receptor. The 5-HT1A receptor is associated with the regulation of mood and the control of the autonomic nervous system. Specific stimulation of the 5-HT1A receptor can produce sympathetic inhibition, decrease blood pressure and heart rate, while stimulation of the 5-HT2A receptor can generate sympathomimetic effects such as increased heart rate, vasomotor tone, and blood pressure. The functional and experiential effects of most psychedelics on humans are primarily mediated through activation of the 5-HT2A receptor.
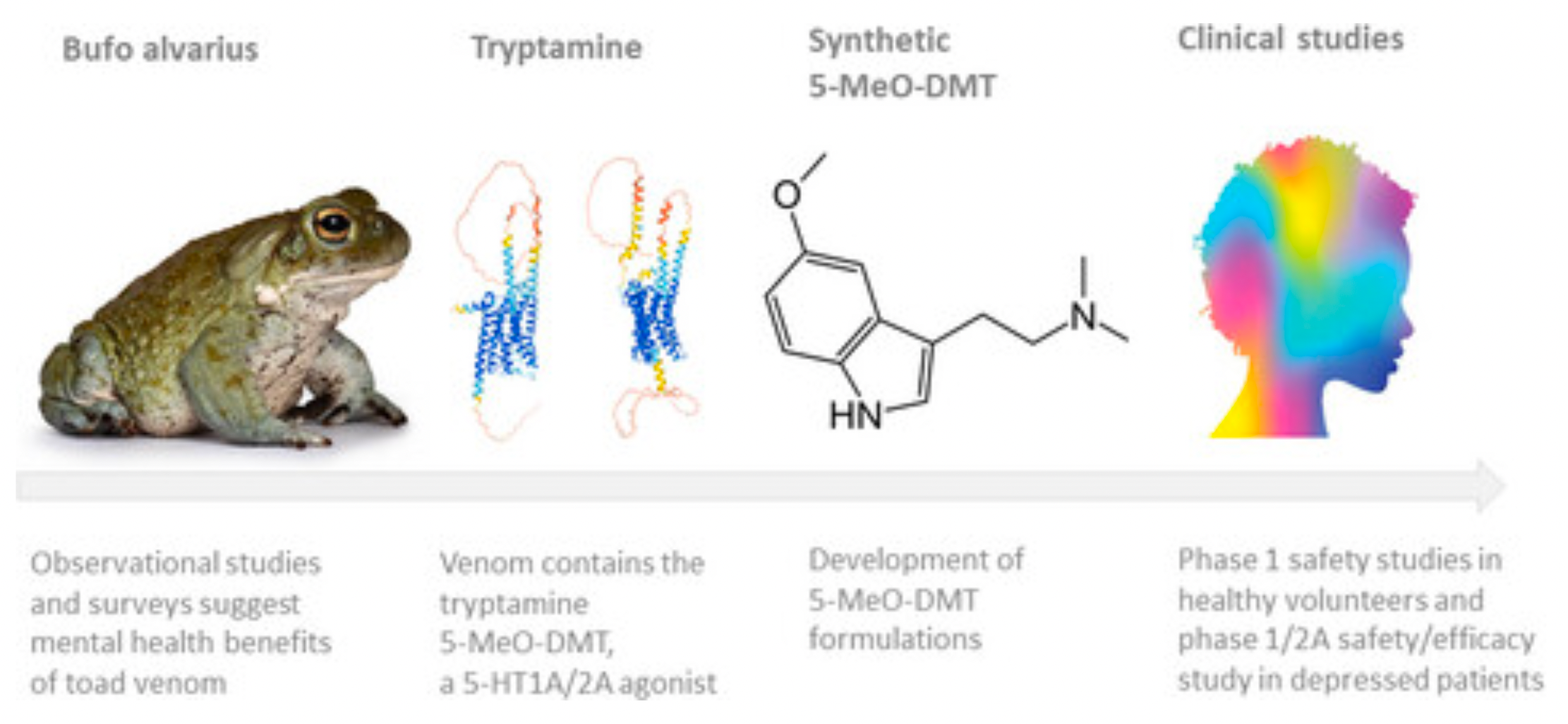
A single escalating dose of 5-MeO-DMT can reliably induce a "peak" experience, which is considered a core predictive marker of the therapeutic effects of psychedelics. A single exposure to 5-MeO-DMT can rapidly and sustainably alleviate symptoms of depression, anxiety, and stress. Additionally, 5-MeO-DMT can stimulate neuroendocrine functions, immunomodulation, and anti-inflammatory processes, all of which contribute to improvements in mental health. Compared to longer-acting psychedelic drugs, 5-MeO-DMT has a rapid onset and short duration of effects, which may make it more suitable for individual dose-finding strategies. Therefore, many biopharmaceutical companies show a strong interest in the development of 5-MeO-DMT formulations, with the primary clinical indication being depression.
According to the Phase 1 clinical trial data released by Beckley Psytech, BPL-003 has demonstrated good safety and tolerability profiles, along with dose-proportional PK/PD curves.
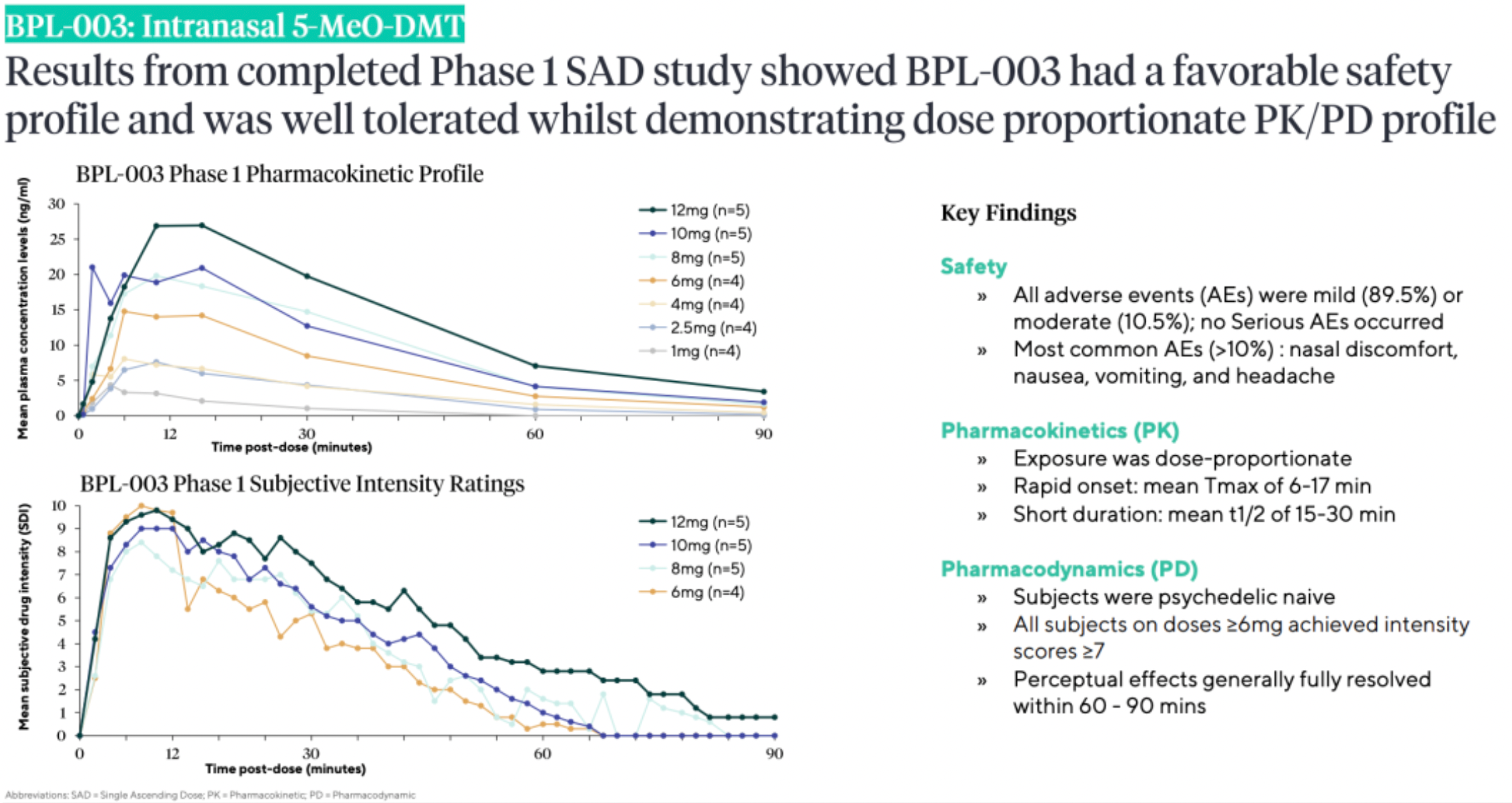
In the completed Phase I trial, the medium and high doses of BPL-003 reliably induced profound psychedelic experiences with rapid onset of psychedelic effects within minutes and dissipation of all perceptual effects within 60-90 minutes.
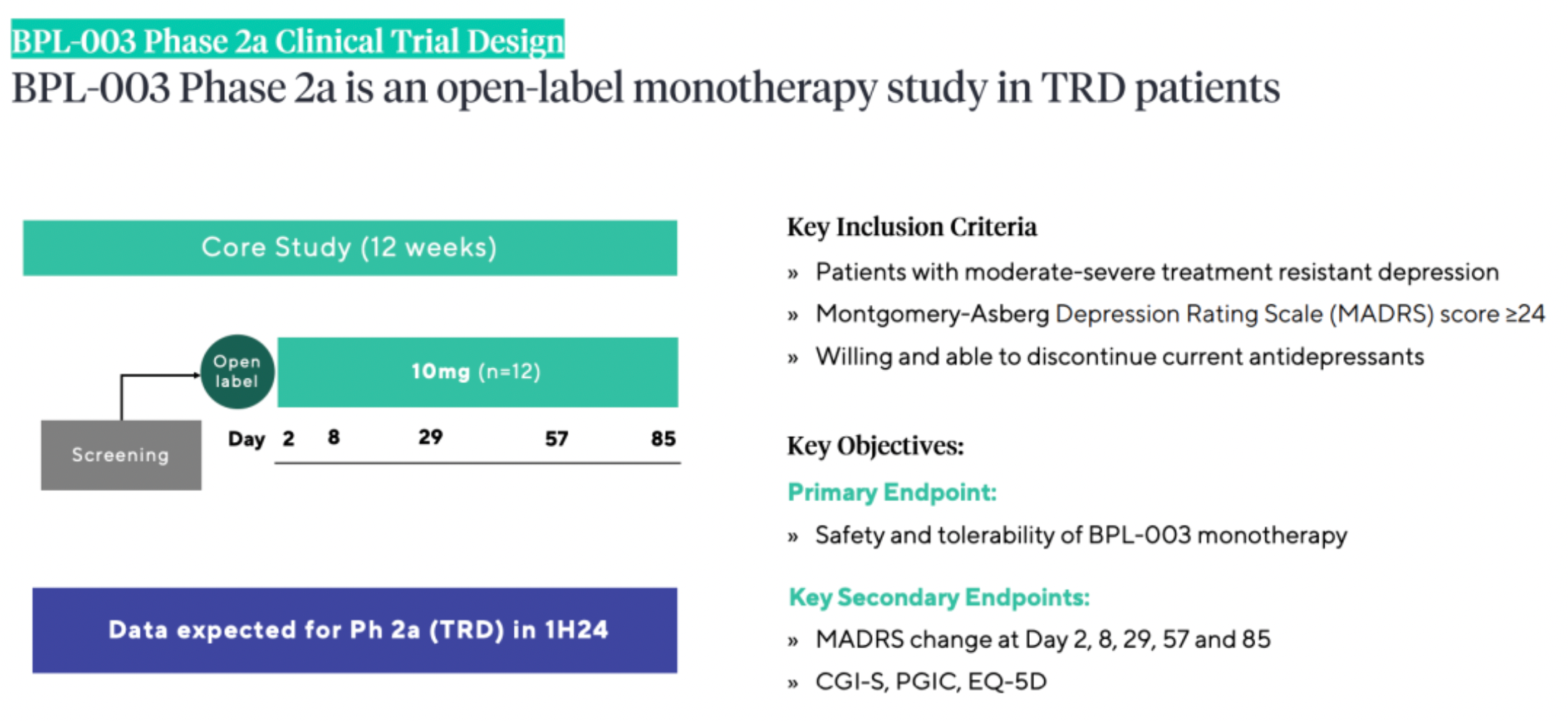
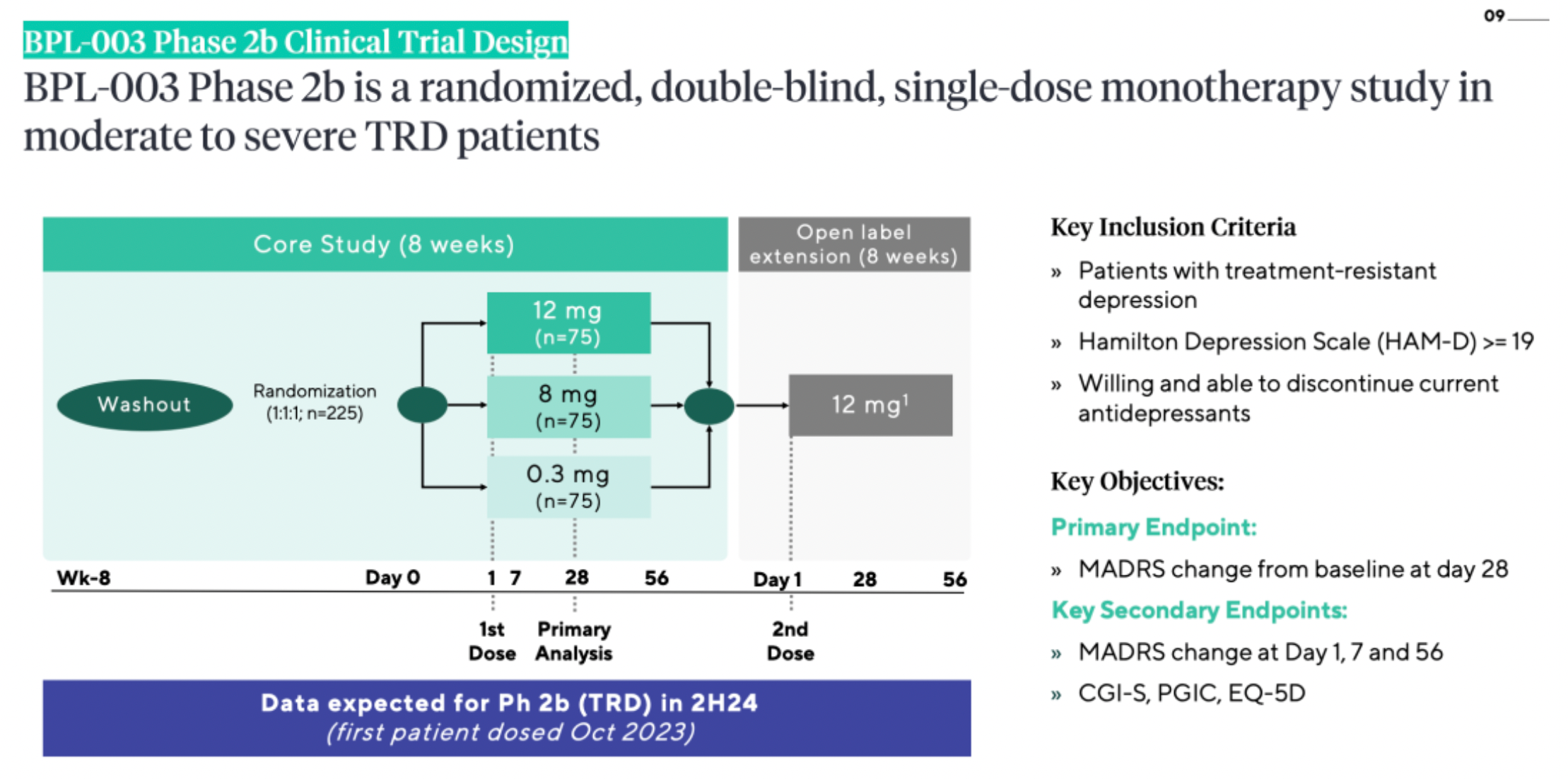
Beckley Psytech is conducting Phase 2a and 2b clinical studies of BPL-003 for patients with treatment-resistant depression, including cases of Treatment-Resistant Depression (TRD) and Alcohol Use Disorder (AUD), with three clinical trials underway. In addition to the Phase 2b TRD study expected to be completed in the second half of 2024, BPL-003 is also being investigated in two small-scale Phase 2a open-label studies in TRD and AUD, with data anticipated in the first half of 2024 and mid-2024, respectively.
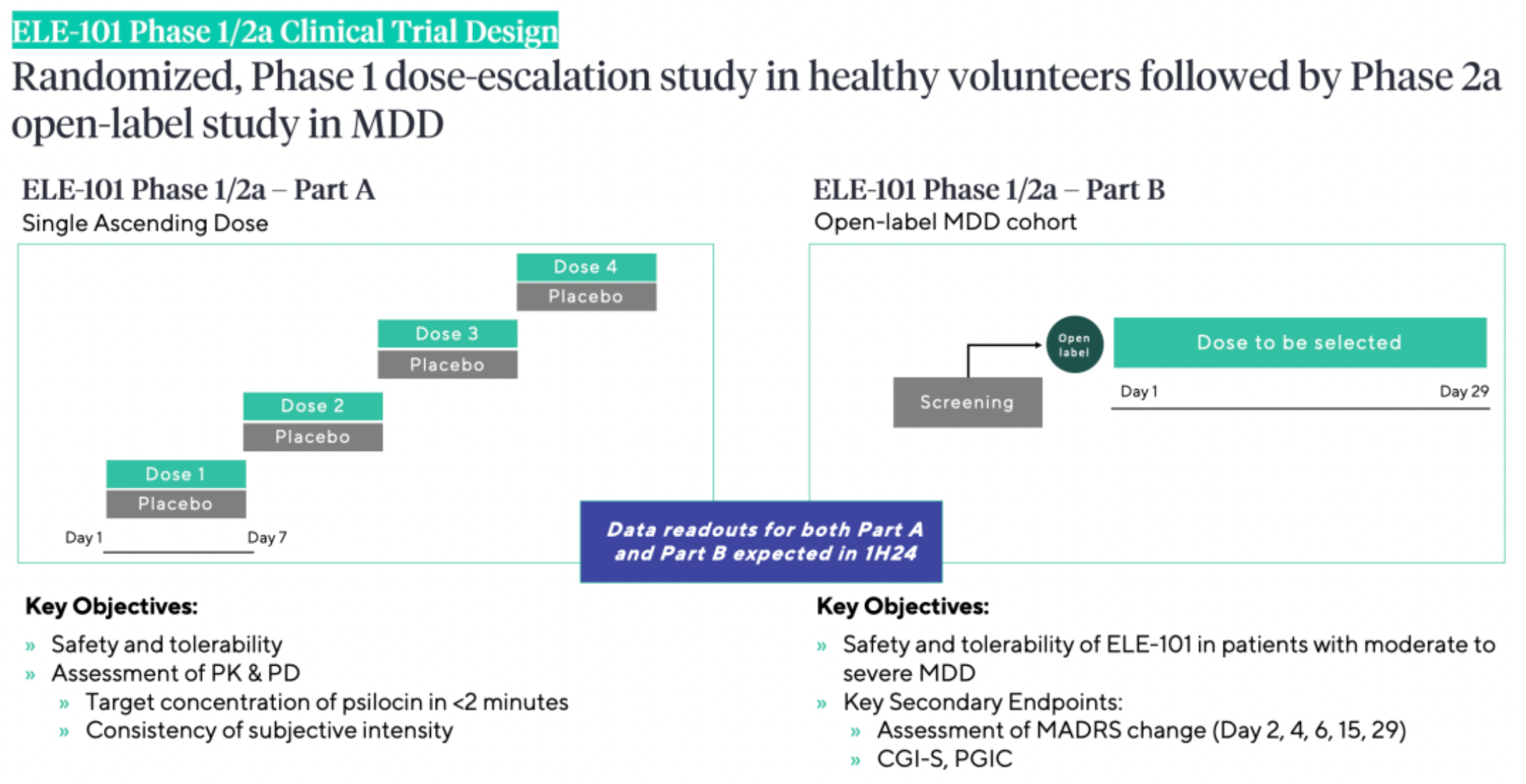
ELE-101 is a novel intravenous formulation of psilocin (4-HO-DMT). Psilocin is the pharmacologically active metabolite of psilocybin, found in psychedelic mushrooms. Due to its structural similarity to serotonin, psilocin is capable of activating serotonin receptors (5-HT1A, 5-HT2A, and 5-HT2C) with affinity in the central nervous system. Activation of the 5-HT2A receptor by psilocin results in increased cortical activity via glutamate excitatory postsynaptic potentials, whereas activation of the 5-HT1A receptor inhibits the activity of pyramidal cells. Additionally, psilocin may produce peripheral effects involving serotonergic receptors. In humans, the psychoactive effects of psilocin, similar to those of hallucinogens, can be observed within 20-30 minutes of ingestion, including visual hallucinations, enhanced auditory perception, and incoordination. Other autonomic effects mediated by psilocin include increased heart rate, elevated blood pressure, pupil dilation, tremors, and elevated body temperature.
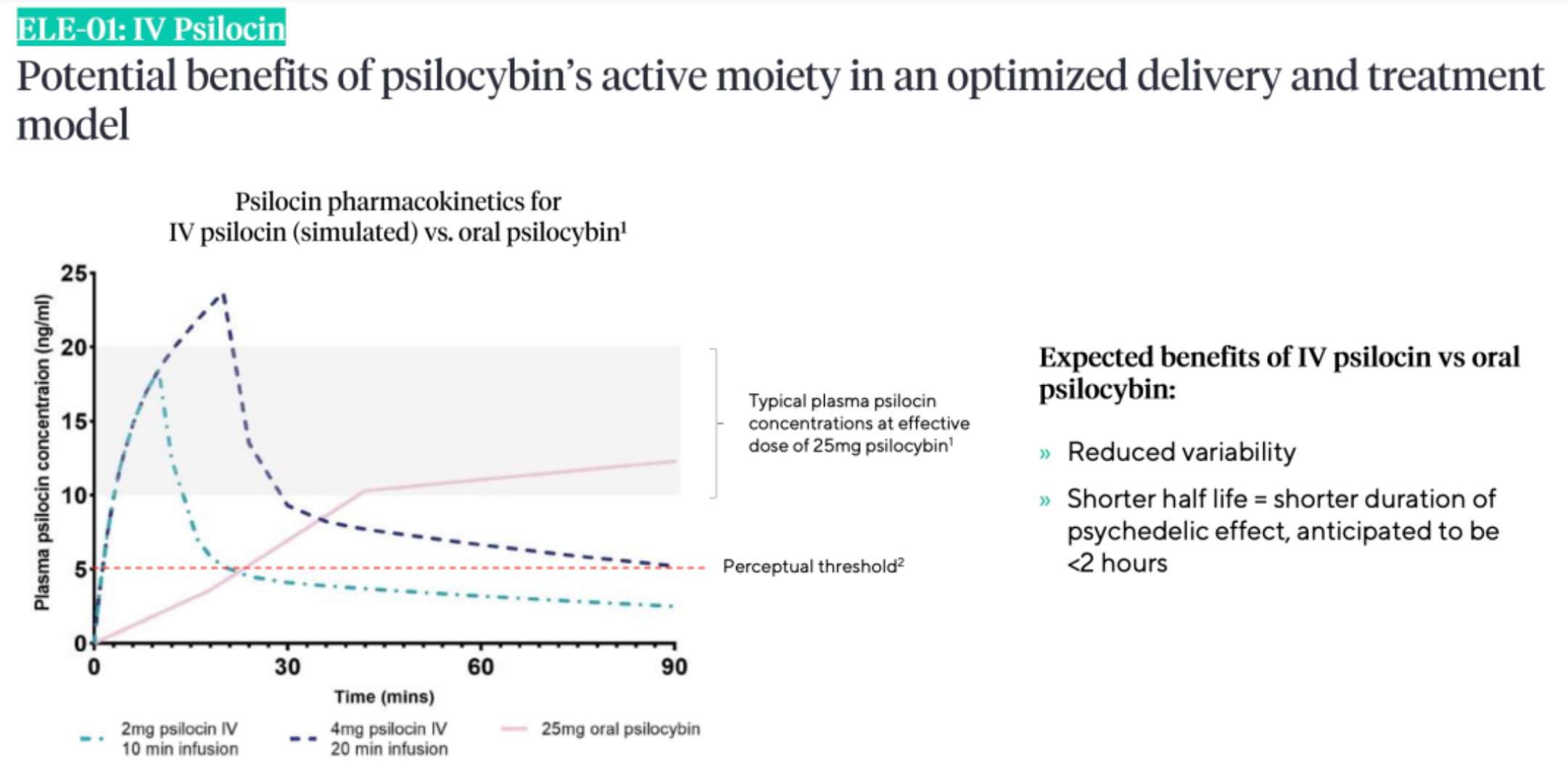
According to research data published by Beckley Psytech, intravenous administration of psilocin as compared to oral psilocybin has shown potential benefits in pharmacokinetic studies, such as reduced variability of the drug and a shorter half-life.
Beckley Psytech is conducting phase 1 and 2a clinical studies for ELE-101 as a treatment for severe depression. Psilocybin has demonstrated notable antidepressant effects in various clinical trials. The treatment paradigm of ELE-101 is more stable, more controllable, and shorter in duration (no more than 2 hours), hence the compound has the potential to offer the therapeutic benefits of psilocybin. Preliminary results for the ELE-101 phase 1/2a study are expected in the first half of 2024.