Atara Biotherapeutics Presents Early CAR T Therapy Research on CD19 at ISCT 2024
Atara Biotherapeutics, Inc., a leader in T-cell immunotherapy, employs its cutting-edge allogeneic Epstein-Barr virus T-cell platform to develop novel therapies for patients with cancer and autoimmune diseases. Recently, the company released preclinical data showcasing the potential of ATA3219, an allogeneic, anti-CD19 chimeric antigen receptor T-cell therapy candidate, in addressing B-cell-driven autoimmune conditions.
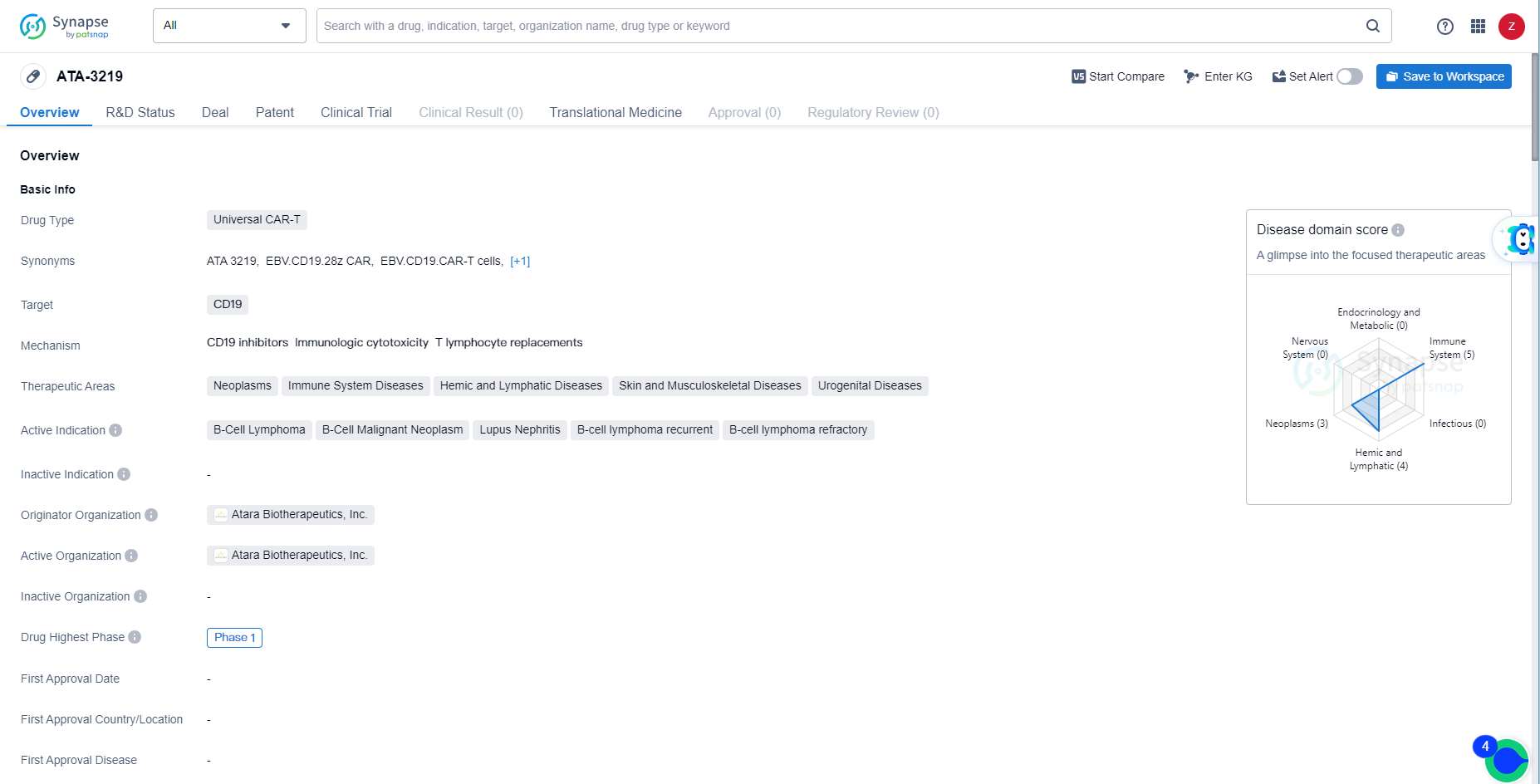
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Studies show that ATA3219 has a comparable cytotoxic function and potency but induces lower levels of pro-inflammatory cytokines than autologous benchmark CD19 CAR T cells. This information will be presented at the International Society for Cell & Gene Therapy 2024 Annual Meeting, taking place from May 29 to June 1, 2024, in Vancouver, Canada.
ATA3219 consists of allogeneic CD19-directed CAR EBV T cells, optimized for ready-to-use applications. It incorporates various clinically validated technologies, including a modified CD3ζ signaling domain that maintains potent effector function while moderating activation and inflammation, a less differentiated phenotype that promotes robust expansion and persistence, and the absence of gene editing to the endogenous T-cell receptor, which serves as a vital T-cell survival signal.
"After seeing promising initial clinical outcomes with autologous CD19 CAR T cells in autoimmune patients, we recognize an opportunity to enhance long-term efficacy, reduce toxicity, and simplify treatment through our advanced allogeneic CD19 CAR T cells," said Cokey Nguyen, Ph.D., Executive Vice President, Chief Scientific & Technical Officer at Atara.
"We are enthusiastic about presenting encouraging preclinical data showing that ATA3219 effectively depletes B-cells against immune cells from SLE and multiple sclerosis patients. Importantly, ATA3219 is an off-the-shelf option with a favorable inflammatory profile, which could result in lower toxicity and better tolerability in clinical applications. We look forward to continued evaluation of ATA3219 for treating non-Hodgkin's lymphoma, lupus nephritis, and in the newly announced cohort expansion for severe SLE without lymphodepletion," Nguyen concluded.
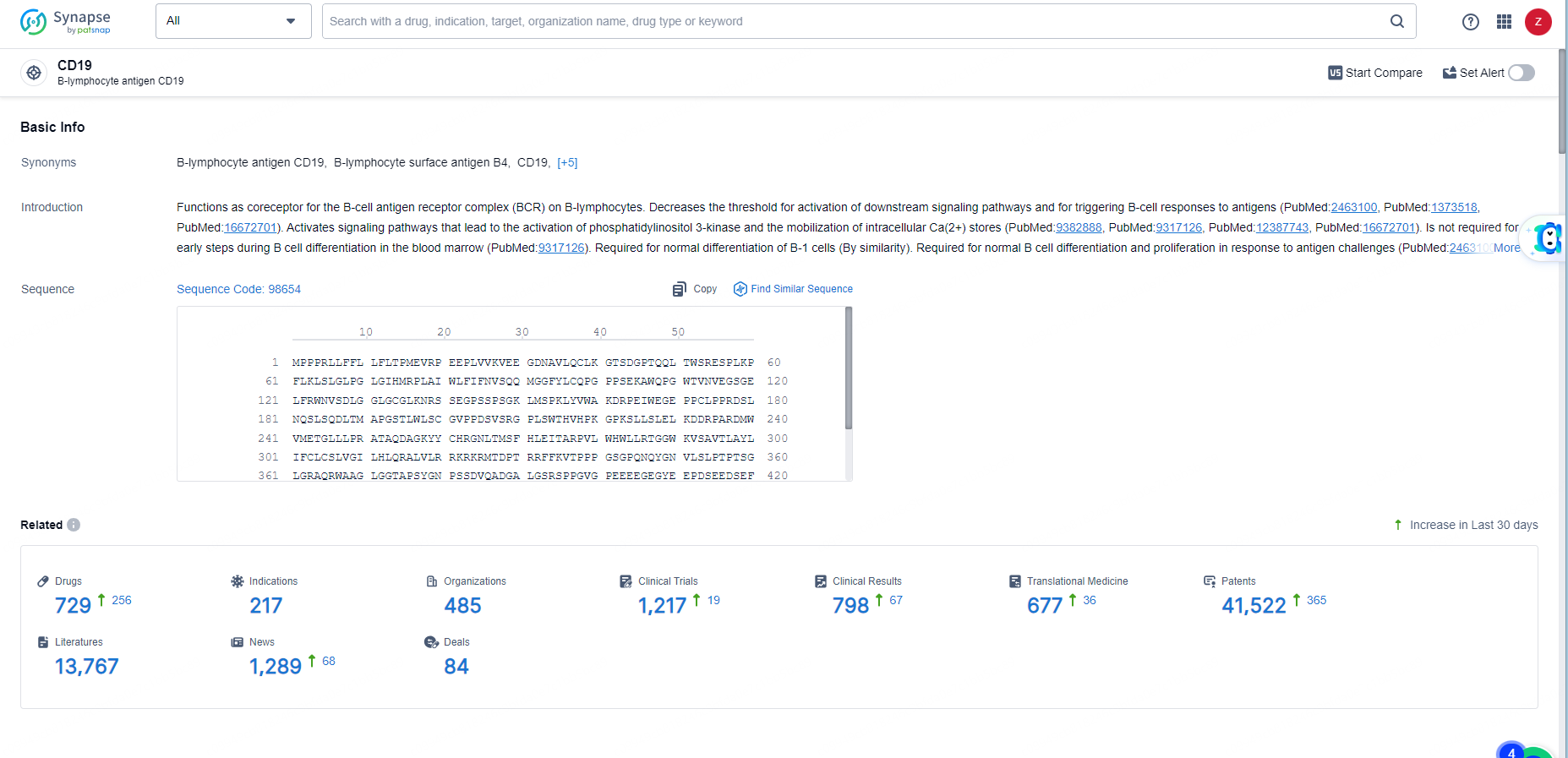
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 3, 2024, there are 729 investigational drugs for the CD19 targets, including 217 indications, 485 R&D institutions involved, with related clinical trials reaching 1217, and as many as 41522 patents.
ATA-3219 is a Universal CAR-T drug targeting CD19 with a focus on treating B-cell related malignancies and autoimmune conditions. Its current status at Phase 1 of development indicates ongoing progress in clinical testing, and further advancements in its development may hold promise for patients with the specified therapeutic areas and active indications.