FDA Grants Approval for First Interchangeable Biosimilar to Treat Two Uncommon Conditions
The U.S. Food and Drug Administration has granted approval to Bkemv (eculizumab-aeeb) as the first interchangeable biosimilar to Soliris (eculizumab) for the treatment of specific rare conditions. Bkemv is authorized for the following treatment uses, which match those presently approved for Soliris.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Sarah Yim, who is the director of the Office of Therapeutic Biologics and Biosimilars at the FDA’s Center for Drug Evaluation and Research, stated, “Many serious conditions are life-threatening and lack effective treatments. The FDA is dedicated to promoting the creation of safe and effective interchangeable biosimilar therapies. These innovations can provide greater access for patients with rare diseases that have limited treatment options.”
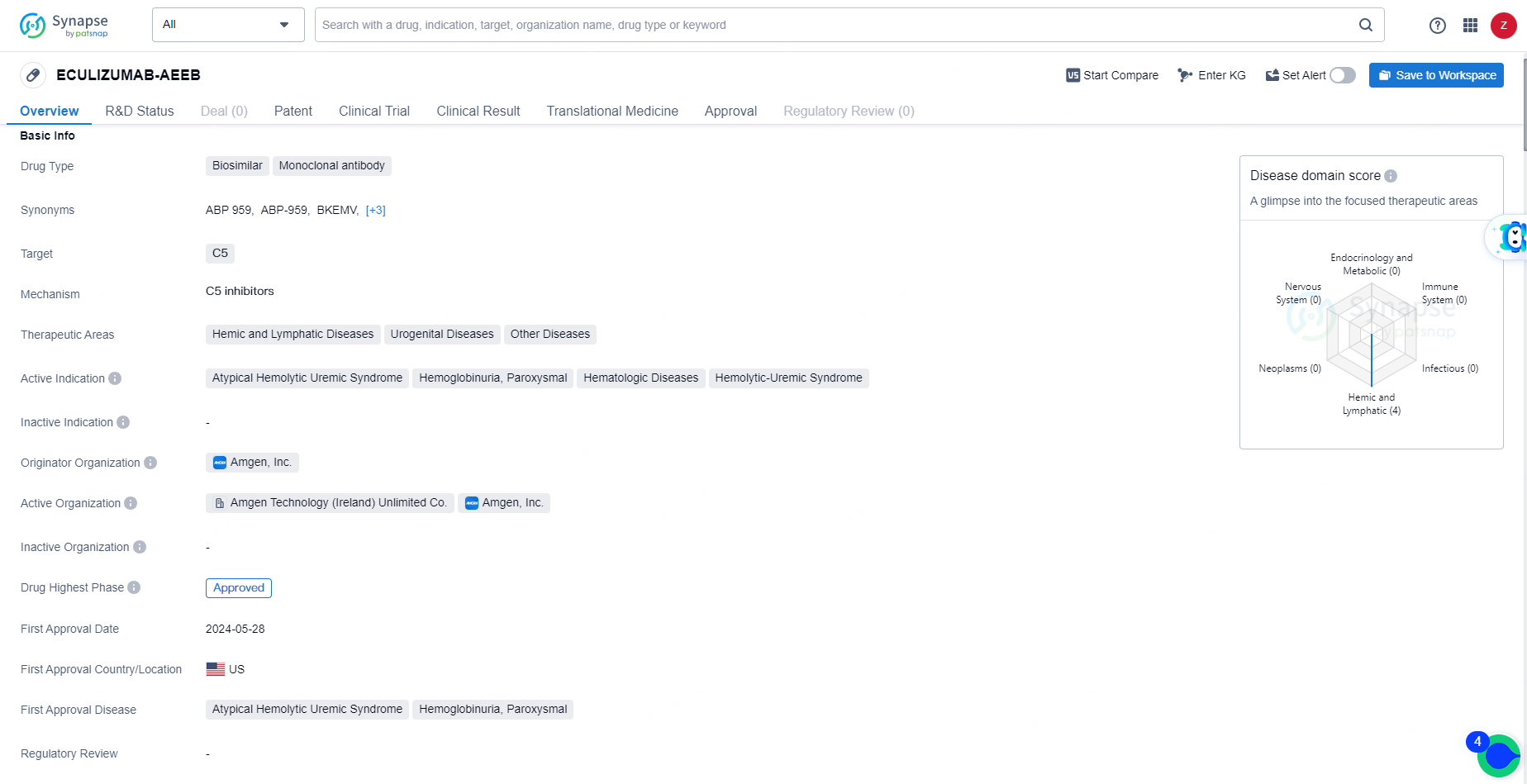
A medical condition is classified as rare if it affects fewer than 200,000 individuals in the United States. PNH (Paroxysmal Nocturnal Hemoglobinuria) and aHUS (atypical Hemolytic Uremic Syndrome) are uncommon disorders marked by the destruction of red blood cells. PNH is associated with symptoms such as anemia, thrombosis, pancytopenia, and dark-colored urine, while aHUS is linked to anemia, thrombocytopenia, and kidney failure.
Bkemv is a monoclonal antibody that targets the complement C5 protein, thereby inhibiting the complement system, an essential part of the immune system. This mechanism prevents the destruction of red blood cells in patients suffering from PNH and aHUS.
Both Bkemv and Soliris carry a Boxed Warning, indicating that eculizumab products can heighten the risk of severe and potentially fatal meningococcal infections caused by Neisseria meningitidis, the bacterium responsible for meningitis and other serious infections. It is crucial that patients complete meningococcal vaccination prior to beginning treatment with either Bkemv or Soliris. Additionally, patients should be monitored for early signs of such infections and promptly evaluated if symptoms arise.
Bkemv is available solely through a special program known as the Bkemv Risk Evaluation and Mitigation Strategy (REMS). This is a drug safety program mandated by the FDA for certain medications with serious safety risks, ensuring that the benefits of the medication surpass its risks. As of now, Bkemv is the 53rd biosimilar approved in the United States, and out of these, 13 are approved as interchangeable biosimilars.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
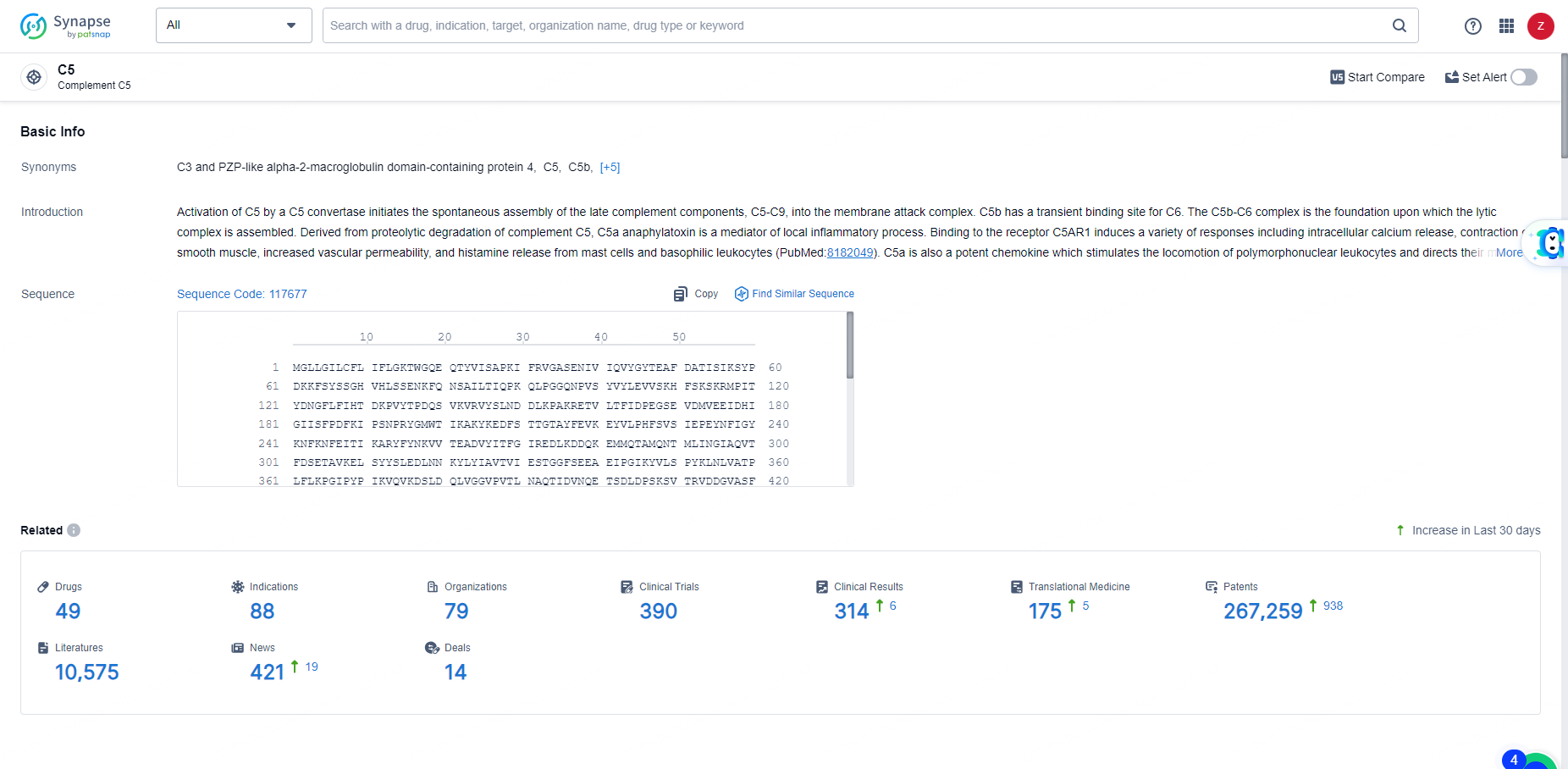
According to the data provided by the Synapse Database, As of May 31, 2024, there are 49 investigational drugs for the C5 target, including 88 indications, 79 R&D institutions involved, with related clinical trials reaching 390, and as many as 267259 patents.
The approval of biosimilar and interchangeable biosimilar products furthers the FDA’s longstanding commitment to support a competitive marketplace for biological products and increase patient access to more affordable treatment options.
ECULIZUMAB-AEEB is a biosimilar drug classified as a monoclonal antibody. The originator organization of ECULIZUMAB-AEEB is Amgen, Inc. It targets the C5 protein and is indicated for the treatment of various hemic and lymphatic diseases, urogenital diseases, and other related conditions. The drug is specifically approved for the treatment of atypical hemolytic uremic syndrome, hemoglobinuria, paroxysmal, and other hematologic diseases.