Bio-Thera and STADA Sign Exclusive Deal for Golimumab Biosimilar in Europe and UK
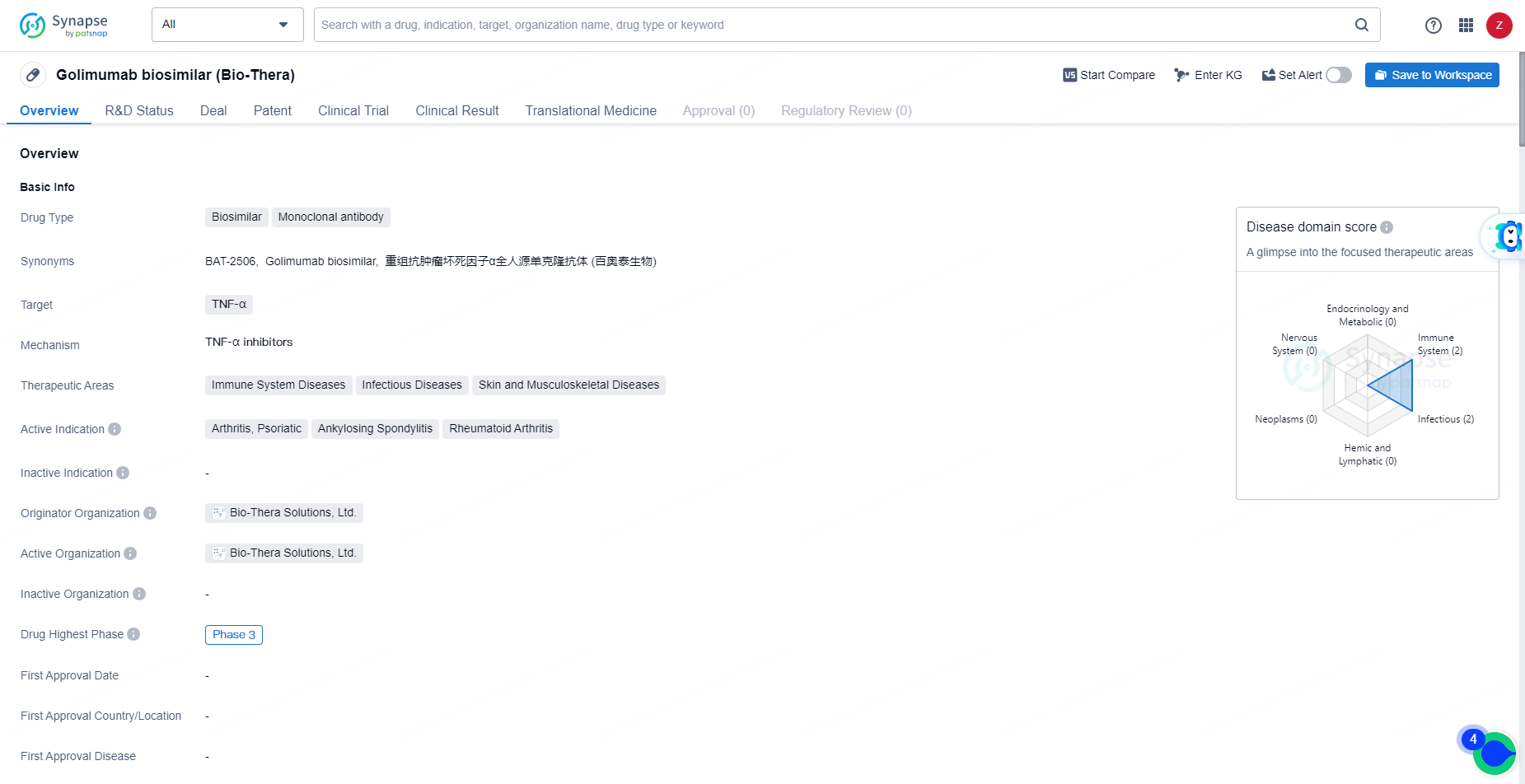
Bio-Thera Solutions, a company in the biopharmaceutical sector that has advanced to the commercial phase and is dedicated to innovative therapeutic advancements and biosimilars, has entered into an exclusive agreement with the global healthcare company STADA Arzneimittel AG, renowned for its expertise in specialty, generic, and consumer health products. The partnership involves the commercialization and licensing of BAT2506, a biosimilar of Simponi® (golimumab).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
According to the contract, Bio-Thera Solutions will manage the development, production, and supply chain of BAT2506. STADA Arzneimittel AG has been granted exclusive rights for the drug's commercialization throughout the European Union, the UK, Switzerland, and certain other areas. Bio-Thera will receive an upfront payment of US$10 million and could earn up to an additional US$147.5 million contingent upon achieving specific development and sales targets.
"STADA is a prominent biosimilar company in Europe, and we are thrilled to partner with them on BAT2506," stated Dr. Shengfeng Li, CEO of Bio-Thera. "We look forward to a productive alliance with STADA to ensure BAT2506 is available to immunology patients across Europe."
"Golimumab achieved global sales of US$2.2 billion in 2023, with revenues exceeding US$1 billion outside the US, indicating a substantial opportunity to broaden patient access to biological treatments. This aligns with our strategy to expand our immunology portfolio, alongside our current adalimumab and ustekinumab biosimilars. Bio-Thera's significant expertise in biologics, as evidenced by the FDA approval of two of their biosimilars, makes them an excellent partner for STADA," commented Bryan Kim, head of Global Specialty at STADA.
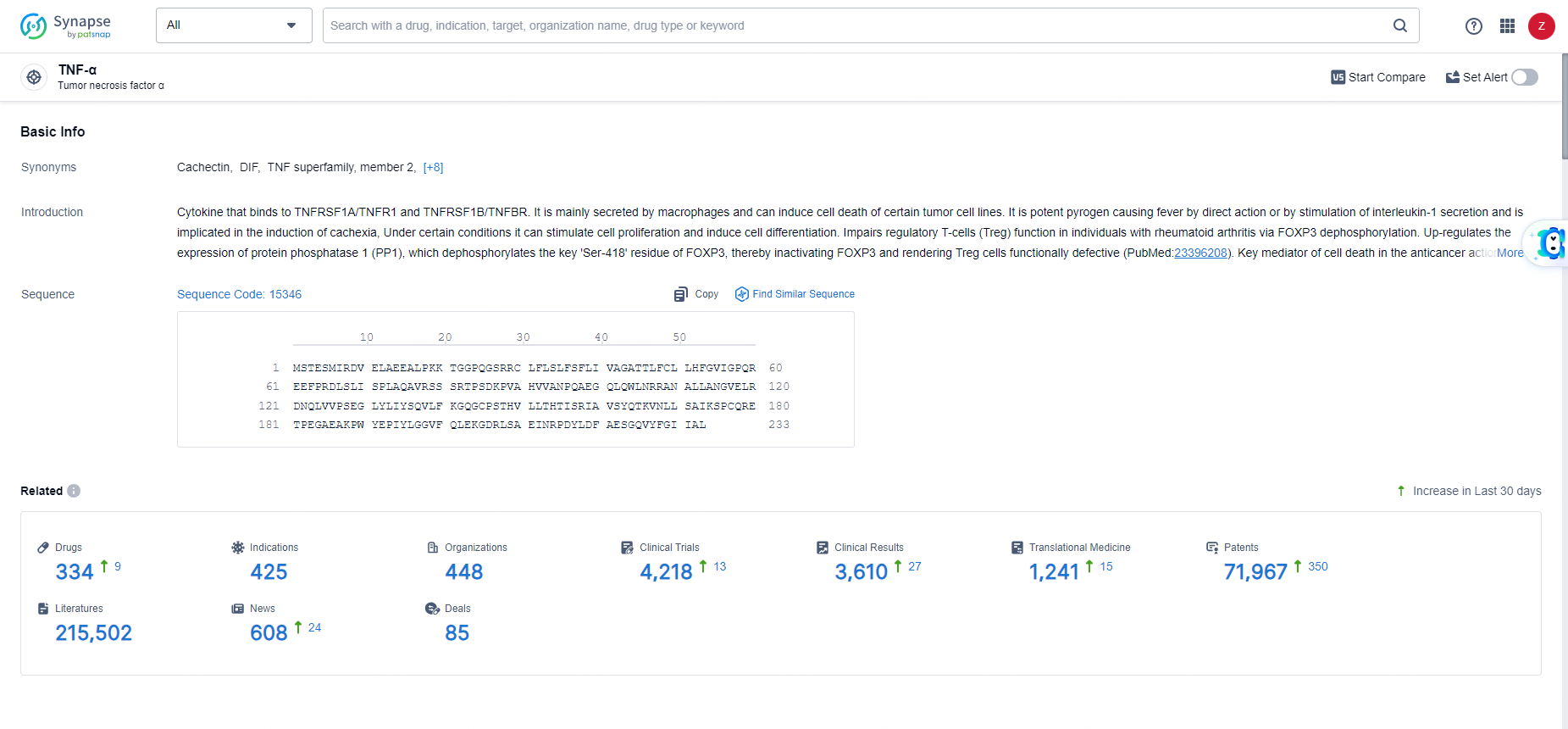
Golimumab is a human IgG1 monoclonal antibody that targets tumor necrosis factor alpha (TNF- α), a crucial molecule in inflammation. The binding of golimumab to TNF-α reduces the levels of C-reactive protein, Interleukin 6, Intercellular Adhesion Molecule 1, Matrix Metalloproteinase 3, and Vascular Endothelial Growth Factor, all of which are indicators of inflammation.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 3, 2024, there are 334 investigational drugs for the TNF-α target, including 425 indications, 448 R&D institutions involved, with related clinical trials reaching 4218, and as many as 71967 patents.
Golimumab biosimilar is a monoclonal antibody biosimilar targeting TNF-α, with active indications in various immune system and musculoskeletal diseases. The drug has reached Phase 3 in its global and Chinese development, indicating its potential as an affordable and effective treatment option for patients with inflammatory and autoimmune conditions.