Outlook Therapeutics Gains EU Approval for Wet AMD Drug LYTENAVA™
Outlook Therapeutics, Inc., a biopharmaceutical firm dedicated to the commercialization and development of ONS-5010/LYTENAVA™ (bevacizumab-vikg; bevacizumab gamma) for retinal disease treatments, has announced the European Commission's approval of Marketing Authorization for LYTENAVA™ (bevacizumab gamma), an ophthalmic bevacizumab formulation for wet AMD treatment in the EU. LYTENAVA™ (bevacizumab gamma) stands as the first and only authorized ophthalmic formulation of bevacizumab for wet AMD treatment within the EU.
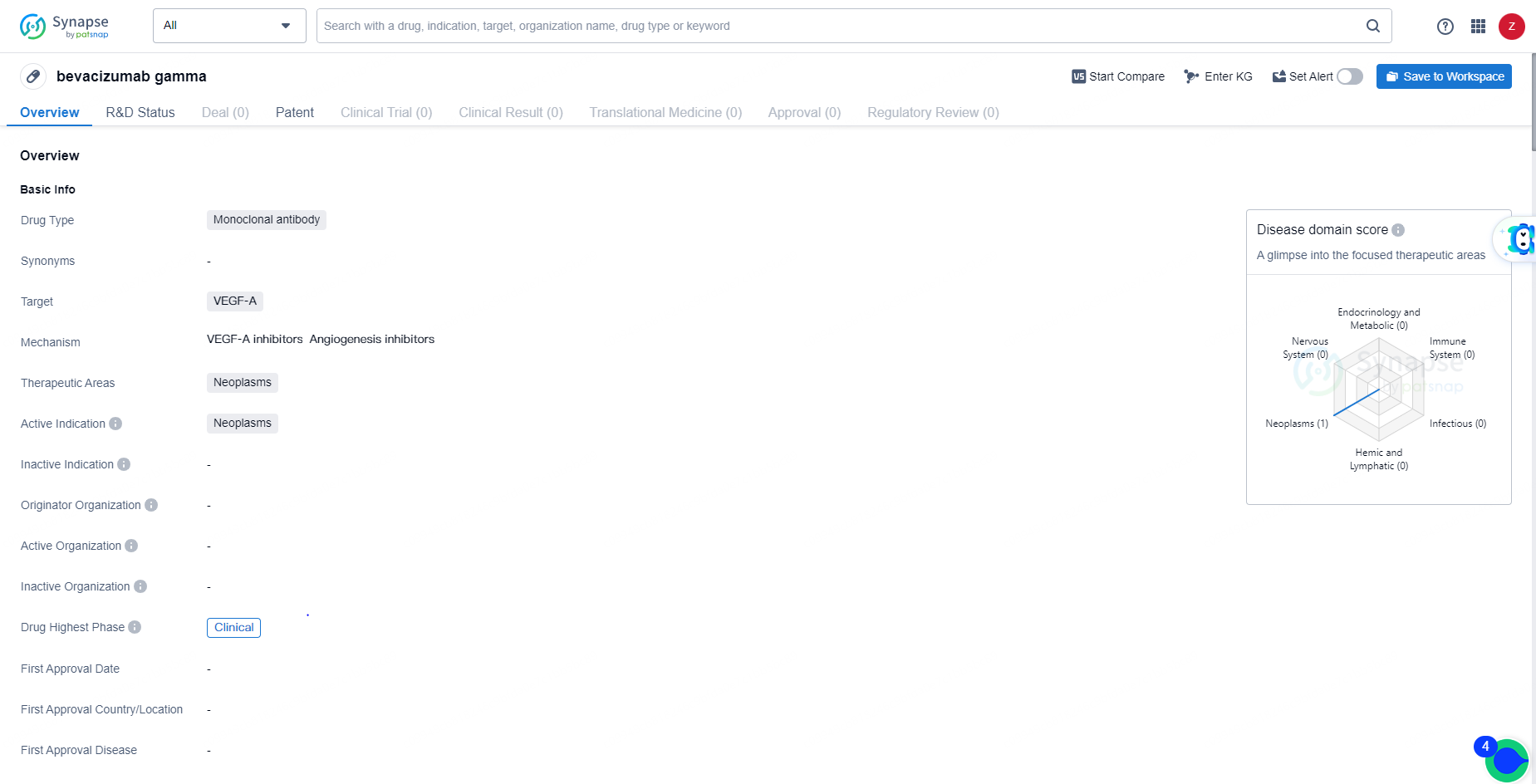
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The European Commission Marketing Authorization application for LYTENAVA™ (bevacizumab gamma) is a mixed filing based on Article 8.3 of Directive 2001/83/EC. This application leverages findings from Outlook's wet AMD clinical program, which includes the completed NORSE ONE, NORSE TWO, and NORSE THREE registration trials, along with various studies and peer-reviewed articles that replace or support some specific tests and studies.
This authorization will automatically apply in all 27 EU Member States and extend to Iceland, Norway, and Liechtenstein within 30 days. Additionally, the Marketing Authorization provides Outlook with ten years of initial market exclusivity for LYTENAVA™ (bevacizumab gamma) in the EU.
"This achievement is a significant milestone for us. We are thrilled to obtain Marketing Authorization for LYTENAVA™ (bevacizumab gamma) in the EU and are preparing for a potential first commercial launch in an EU Member State in the first quarter of 2025. The EU is the second-largest market for wet AMD globally, and we are excited to continue our work to introduce the first and only on-label ophthalmic bevacizumab for wet AMD treatment in the EU," stated Russell Trenary, President and CEO of Outlook Therapeutics.
Jedd Comiskey, Senior Vice President – Head of Europe at Outlook Therapeutics, added, "We believe LYTENAVA™ (bevacizumab gamma) can be a valuable treatment option in the EU ophthalmology sector. We are eager to focus on our anticipated initial launch in the European region. Our team is collaborating closely with our commercialization partner, Cencora, to support our expected EU launch in the first quarter of 2025. We look forward to finalizing our global launch strategy and to an exciting year ahead."
John W. Arena, Senior Vice President of Enterprise Strategy & Solutions at Cencora’s Global Pharma Services, commented, "We have partnered closely with Outlook Therapeutics to develop and implement a global launch strategy and are excited to assist in bringing this therapy to the European market. We will provide the integrated solutions needed for a successful commercial launch and ensure healthcare providers have timely and reliable access to this therapy."
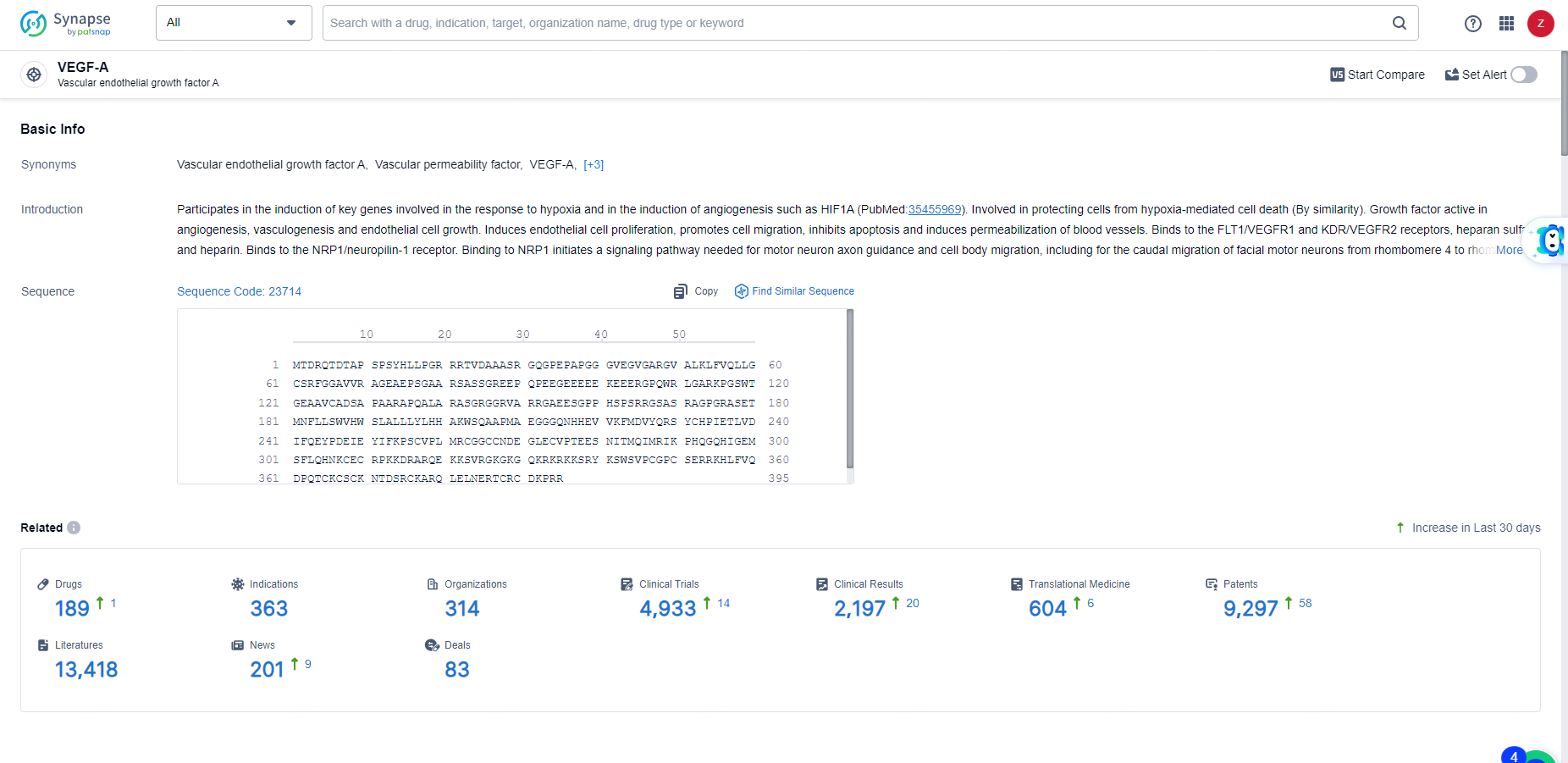
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 31, 2024, there are 189 investigational drugs for the VEGF-A targets, including 363 indications, 314 R&D institutions involved, with related clinical trials reaching 4933, and as many as 9297 patents.
bevacizumab gamma is a monoclonal antibody drug that targets VEGF-A and is being developed for the treatment of neoplasms, particularly in the context of various types of cancer. Its current status in the highest phase of clinical development globally indicates its potential as a future treatment option for patients with neoplastic diseases.