Australian Authorities Approve ImmuneSensor Therapeutics' Phase 1 Trial for cGAS Inhibitor IMSB301
ImmuneSensor Therapeutics, a biotherapeutics firm in clinical stages, is dedicated to creating leading inhibitors and agonists for the cGAS-STING pathway, targeting various peripheral and central nervous system conditions related to inflammation, autoimmunity, and cancer. The company has announced that it has received approval from the Human Research Ethics Committee (HREC) and clearance for Clinical Trial Notification (CTN) from the Australian Therapeutic Goods Administration (TGA) to begin a Phase 1 placebo-controlled, double-blind randomized clinical trial for its primary drug candidate IMSB301 in healthy participants. IMSB301 is a new, orally administered small molecule cGAS inhibitor designed for treating inflammatory and autoimmune disorders.
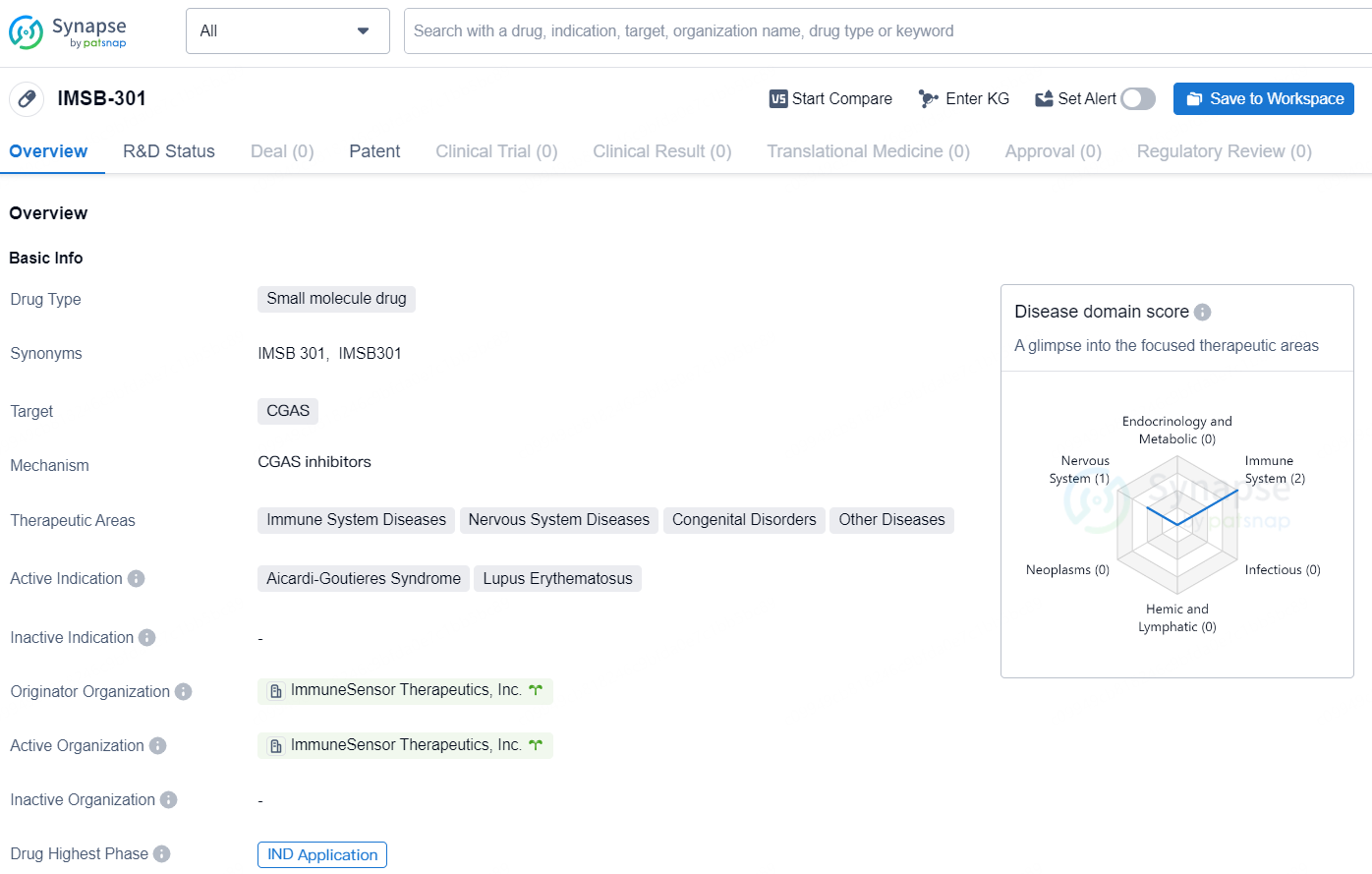
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“Securing HREC approval and CTN clearance for our first clinical trial of the novel cGAS inhibitor marks a major achievement for ImmuneSensor in our mission to provide groundbreaking therapeutic options for individuals suffering from autoimmune and other inflammatory conditions,” stated Tom Dubensky, Ph.D., president and CEO of ImmuneSensor. “The initial dose escalation study of IMSB301 in healthy volunteers will yield vital early safety and target engagement data, enabling swift progression to Phase 1b/2 clinical trials in patients with severe inflammatory disorders, such as Aicardi-Goutières syndrome (AGS), a rare condition driven by persistent activation of the cGAS pathway, as well as defined subgroups of patients with systemic lupus erythematosus (SLE).”
About the IMSB301 Phase 1 Clinical Program
This Phase 1 trial is a randomized, placebo-controlled, double-blind study designed to evaluate the safety, tolerability, and pharmacokinetics (PK) of single and multiple escalating doses of IMSB301, while also confirming target engagement. Healthy volunteers will be recruited into cohorts of eight participants, with two receiving placebo and six receiving IMSB301 at up to five single ascending dose (SAD) levels over two days, followed by up to three multiple ascending dose (MAD) levels. The main goal is to assess safety and tolerability, while PK and target engagement will be measured through an ex vivo whole blood DNA stimulation assay in the MAD group.
About IMSB301
The cGAS-STING pathway is a critical mechanism for detecting host-derived or foreign cytoplasmic DNA resulting from infection, cell damage, or malignancy. In autoimmune diseases, this pathway is constantly activated, fueling inflammation and fostering autoimmunity in both the peripheral and central nervous systems (CNS). IMSB301, an innovative, orally available small molecule, is specifically engineered to inhibit the cGAS enzyme and mitigate pathological inflammation. Preclinical studies have shown that IMSB301 effectively inhibits cGAS enzymatic activity, significantly reducing cytokine production and reversing disease phenotype and premature mortality in a model of AGS. ImmuneSensor is advancing IMSB301 development initially for cGAS-driven Type I interferonopathies, including AGS and SLE. IMSB301 has the potential to address a wide range of therapeutic areas characterized by cGAS-mediated inflammation such as diabetic kidney disease, age-related macular degeneration, and other autoimmune conditions.
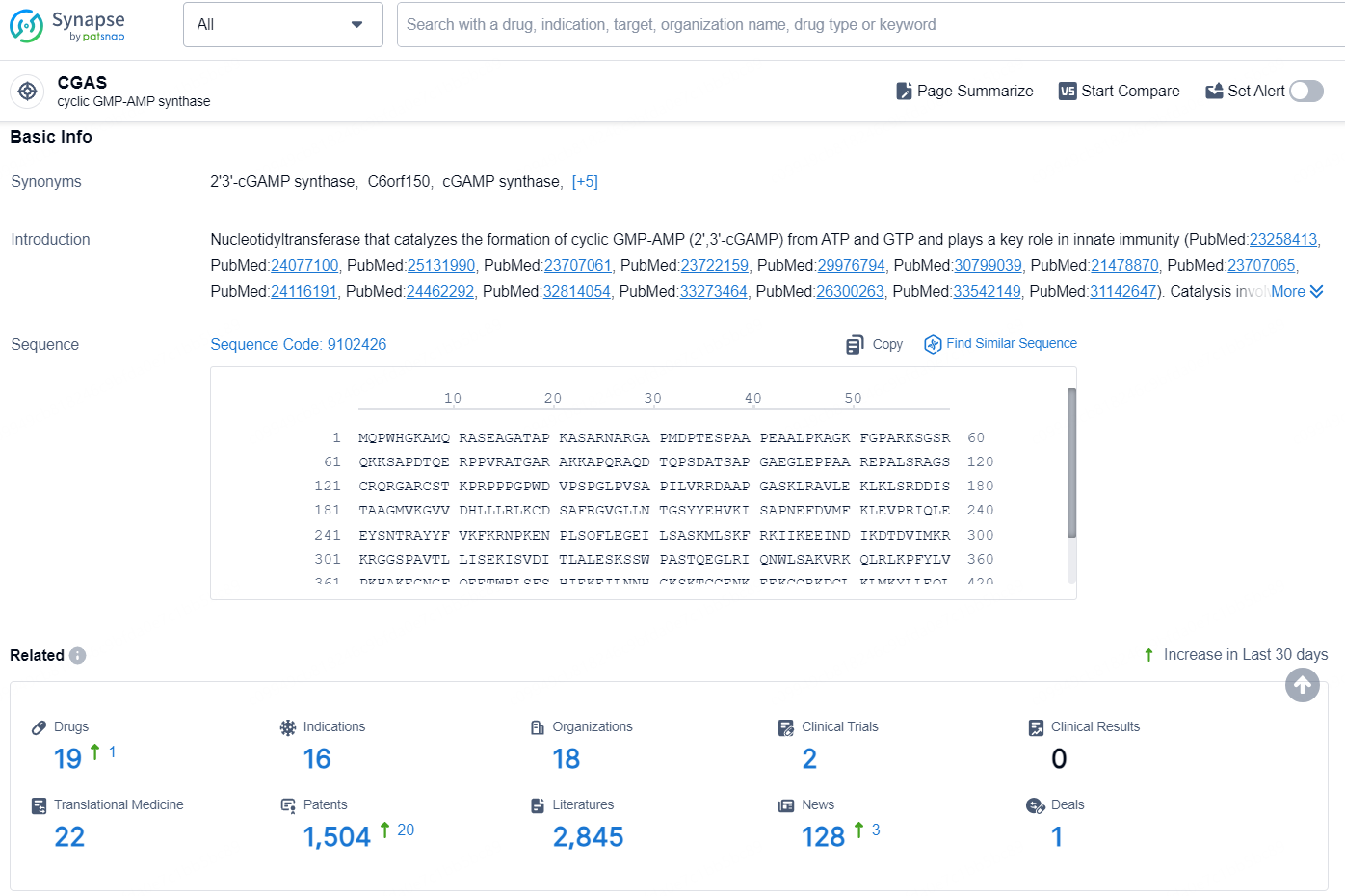
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 22, 2024, there are 19 investigational drugs for the CGAS target, including 16 indications, 18 R&D institutions involved, with related clinical trials reaching 2, and as many as 1504 patents.
IMSB-301 is a small molecule drug developed by ImmuneSensor Therapeutics, Inc., with a primary target of CGAS. This drug has been classified as a small molecule and is currently in the highest phase of development, IND Application. The therapeutic areas for IMSB-301 include Immune System Diseases, Nervous System Diseases, Congenital Disorders, and Other Diseases. The drug is being explored for its potential efficacy in treating Aicardi-Goutieres Syndrome and Lupus Erythematosus.