GlycoNex Completes Phase 1 Trial of Denosumab Biosimilar, SPD8 Successfully
GlycoNex (4168, hereinafter GNX), a biotechnology firm in the clinical stage concentrating on glycan-directed cancer treatments, has declared the successful conclusion of a Phase 1 clinical trial for its denosumab biosimilar, SPD8. This biosimilar was developed in partnership with Mitsubishi Gas Chemical Company, Inc. (MGC). The trial results allow GNX to proceed to Phase 3 clinical trials, targeting the provision of a more affordable alternative to existing denosumab therapy for osteoporosis.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The initial Phase 1 trial, which was conducted with healthy postmenopausal women, indicated that the denosumab biosimilar developed by GlycoNex achieved the primary endpoint of clinical pharmacokinetic equivalence. The study confirmed that the biosimilar’s pharmacokinetic characteristics, safety, and pharmacodynamics were comparable to those of the reference biologic, denosumab.
These encouraging results have positioned GlycoNex to finalize the plans for its Phase 3 clinical program for SPD8, slated to begin in Q4 2024. This upcoming phase will further evaluate the efficacy, safety, and immunogenicity of SPD8 in a larger cohort of osteoporosis patients. GlycoNex is collaborating with regulatory bodies to ensure the trial's design complies with all the necessary standards for thoroughly assessing the biosimilar's clinical performance.
Mei-Chun Yang, CEO of GlycoNex, stated, “Completing the SPD8 Phase 1 study is a significant achievement in developing a denosumab biosimilar to treat osteoporosis and reflects GlycoNex’s mission to make high-quality biologic therapies more accessible and cost-effective. As we advance to Phase 3, we are dedicated to providing a safe and effective denosumab biosimilar that can improve patient outcomes and reduce the financial impact on osteoporosis patients and global healthcare systems.”
In 2023, global sales for two denosumab-based medications reached 4 billion USD and 2.1 billion USD, respectively. With the growing elderly population and increased awareness about osteoporosis risks, the market for denosumab is expanding. Besides targeting the Japanese market, GlycoNex is pursuing international development plans to offer better treatment options to osteoporosis patients globally.
In partnership with Mitsubishi Gas Chemical (MGC), GlycoNex is advancing the development and production of SPD8. Recently, MGC and GlycoNex completed the Phase 1 clinical trial of SPD8 in Japan, marking a significant milestone in its progression.
Ms. Yang added, “We are excited by the progress of SPD8 and the potential to create an additional revenue stream for GlycoNex, while exhibiting our capacity in engineering monoclonal antibodies (mAbs) for various disease indications. Our pipeline continues to grow with the ongoing development of our GNX102 antibody-drug conjugate (ADC), which merges a proprietary mAb with cytotoxic drugs to treat multiple high-prevalence cancers.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
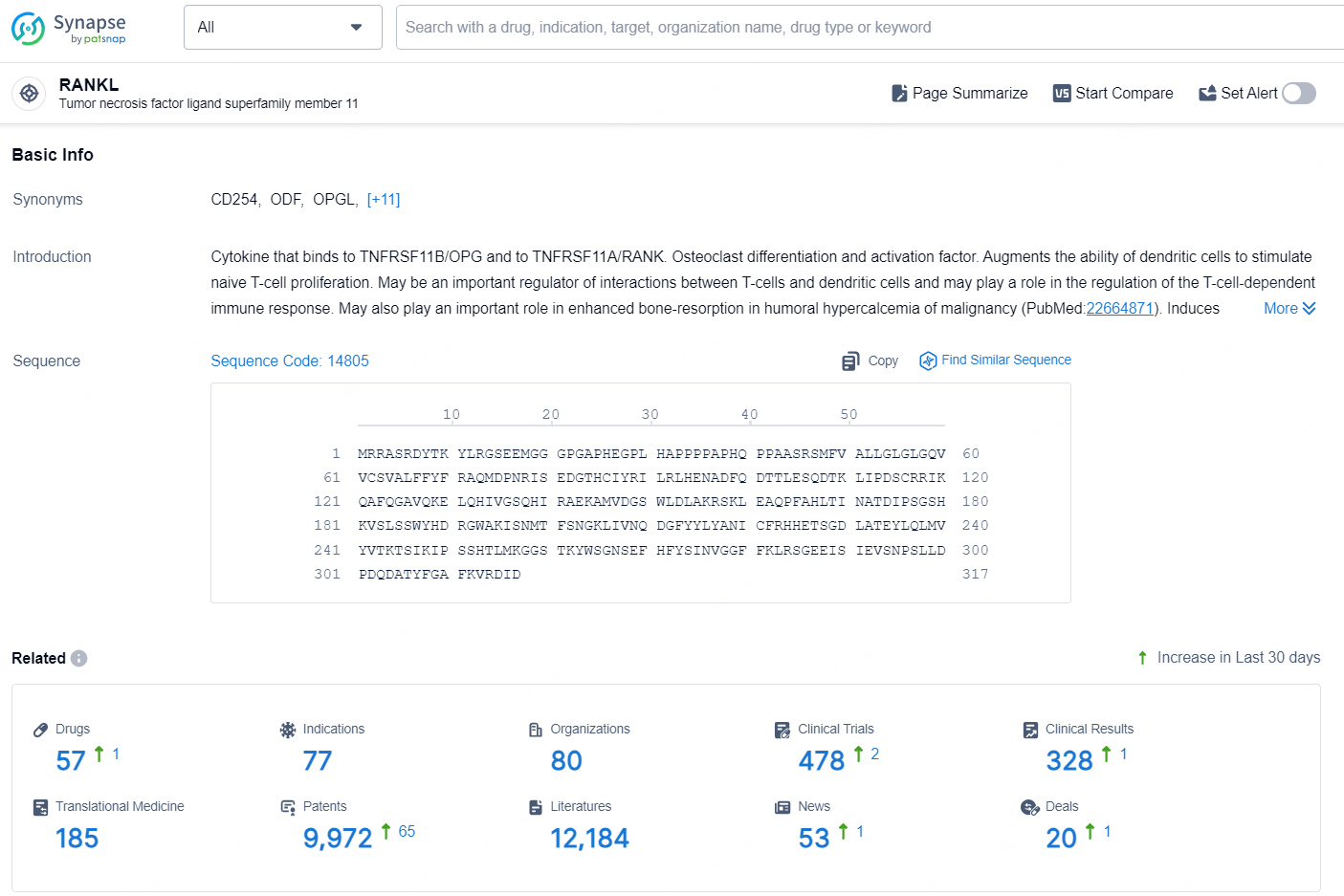
According to the data provided by the Synapse Database, As of August 21, 2024, there are 57 investigational drugs for the RANKLtargets, including 77 indications, 80 R&D institutions involved, with related clinical trials reaching 478, and as many as 9972 patents.
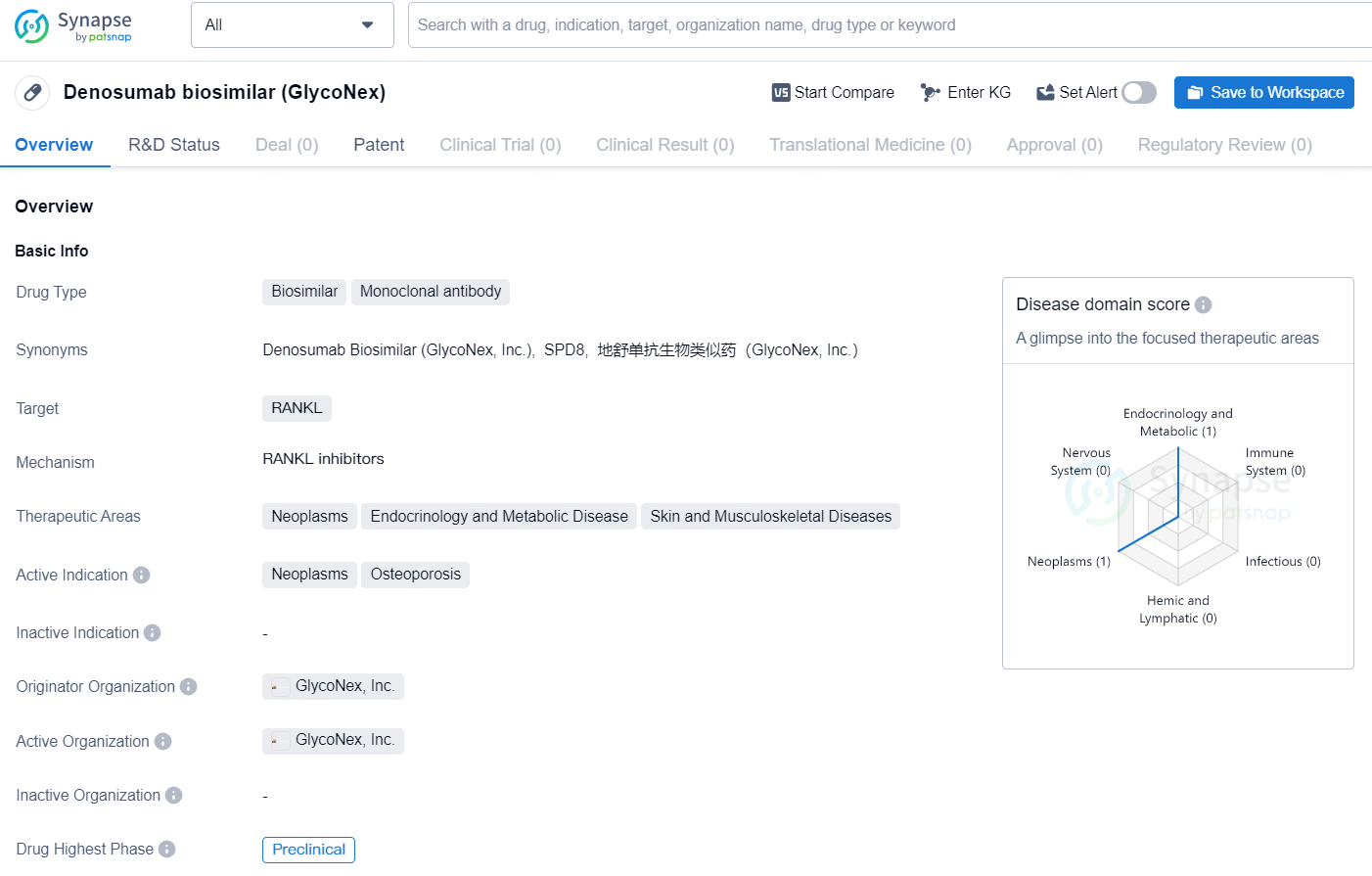
Denosumab biosimilar (GlycoNex) is a biosimilar drug with a monoclonal antibody drug type that targets RANKL. It is intended for use in therapeutic areas related to neoplasms, endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. The active indications for Denosumab biosimilar (GlycoNex) include neoplasms and osteoporosis.