Rising Tides in ADCs: Clinical Progress and Regulatory Outlook for Targeted Cancer Therapies
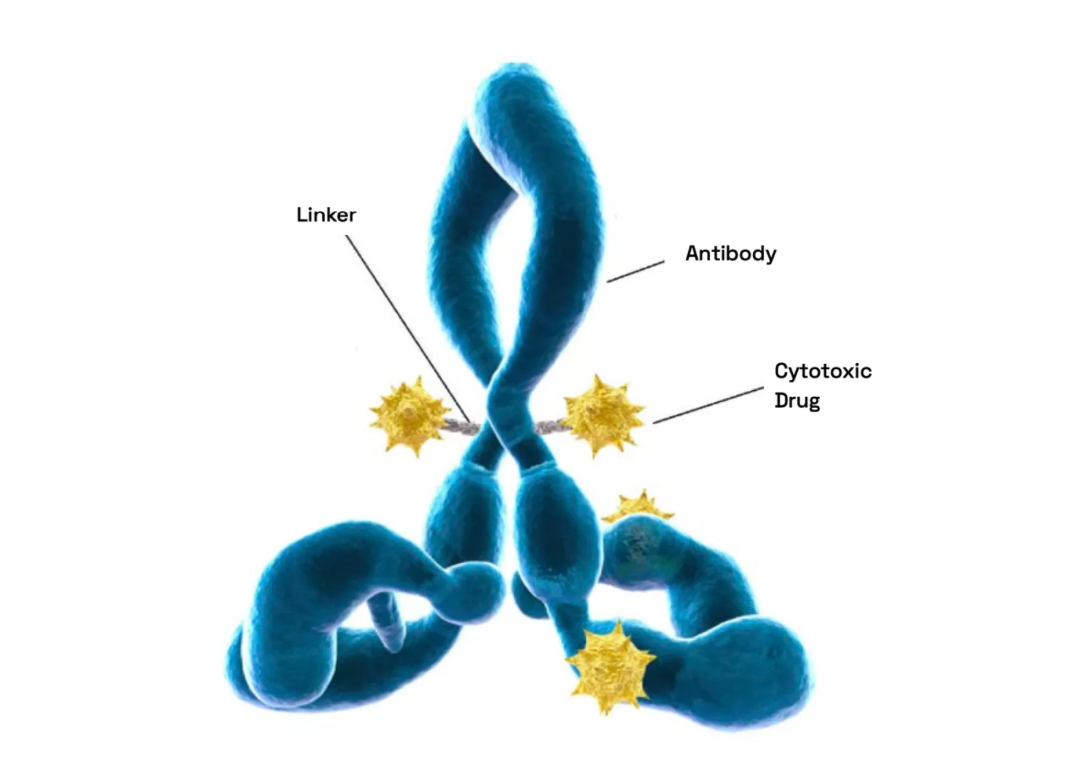
Antibody-Drug Conjugates (ADCs), as a part of precision medicine, target tumor cells by combining the specificity of antibodies with the potent effects of cytotoxins. The design and manufacturing of ADCs pose multifaceted challenges, involving the selection and integration of antigens, antibodies, linkers, and active payloads.
It is projected that by 2028, the revenue generated from approved ADCs and those in Phase III clinical development will reach 26 billion USD.
According to the Synapse database, currently, there are over 1700 ADC therapeutics in the global pipeline, with nearly 200 of them in clinical research stages, and several are in the process of applying for market approval.
Recently, John Wu from the Boston Consulting Group and colleagues published a review article titled "The antibody–drug conjugate landscape" in Nature Reviews Drug Discovery.
The authors analyzed data on 168 antibody-drug conjugates (ADCs) currently in clinical development and found that approximately 85% of these assets are aimed at solid tumor indications, with breast and lung cancers being the most common.
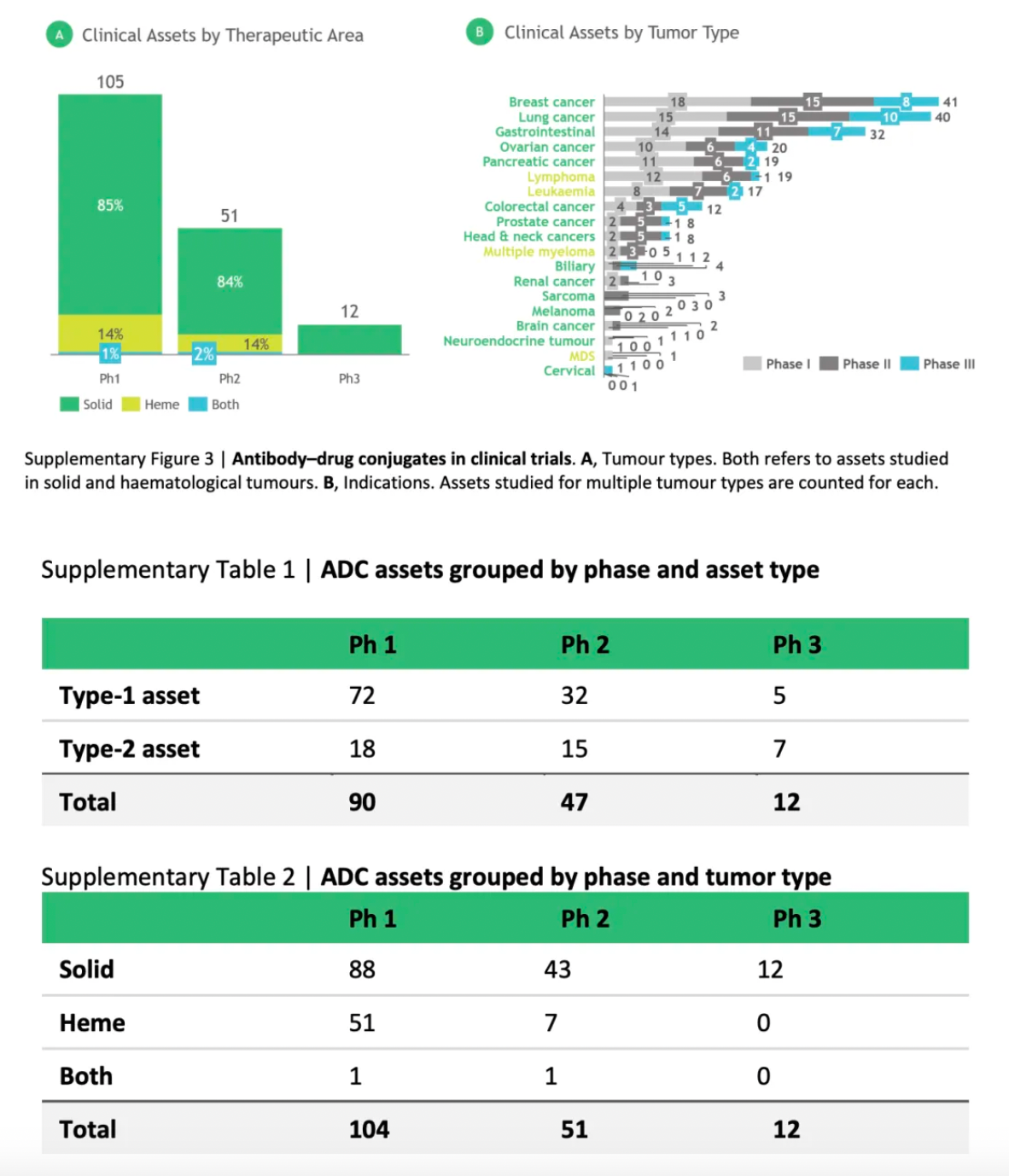
Among the ADCs in phase III clinical trials, about 60% are considered second-generation assets that utilize established target/cargo mechanisms of action (MoA) combined with novel delivery components to achieve best-in-class characteristics. In early-stage development, higher biological risks are more evident; approximately 75% of the phase I/II ADCs are categorized as first-generation assets, featuring new targets and/or payload mechanisms, demonstrating potential as first-in-class drugs.
In 2000, the United States Food and Drug Administration (FDA) approved the first ADC (Antibody Drug Conjugate) drug, Gemtuzumab ozogamicin (brand name Mylotarg), for the treatment of adult patients with Acute Myeloid Leukemia (AML).
Gemtuzumab ozogamicin combines a humanized anti-CD33 monoclonal antibody with the potent cytotoxic drug calicheamicin, delivering the drug directly to the leukemia cells expressing CD33. However, due to the emergence of severe side effects, including abnormal liver function and the so-called Veno-Occlusive Disease (VOD), it was voluntarily withdrawn from the market by the manufacturer in 2010. Nevertheless, considering its efficacy in certain AML patients, following further research and risk assessment, Gemtuzumab ozogamicin was re-approved by the FDA in 2017 for the treatment of newly diagnosed CD33-positive AML in adult patients, as well as in pediatric patients aged two years and older.
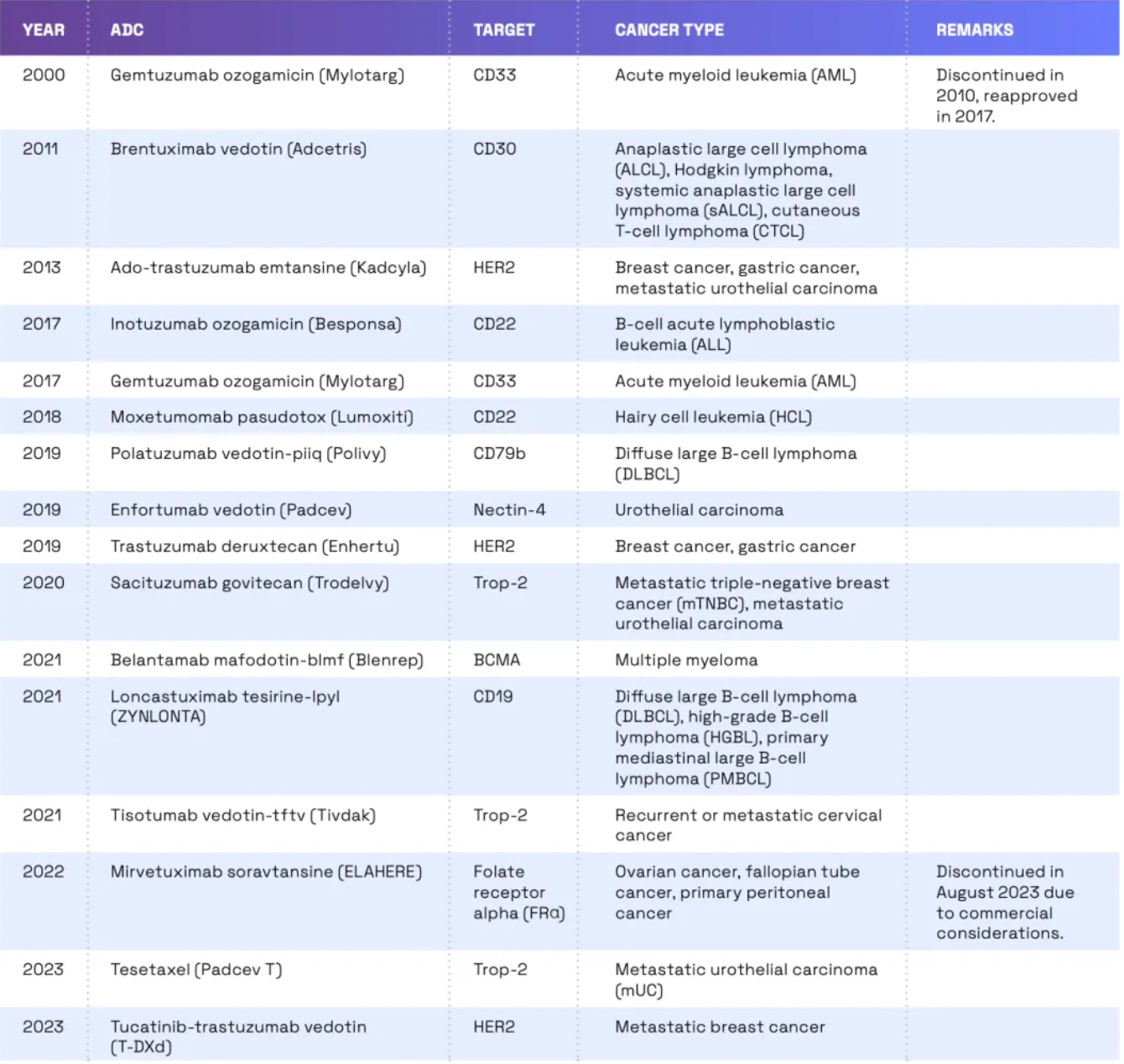
Since 2011, there has been a significant increase in the number of ADCs (antibody-drug conjugates) approved by the FDA. According to the latest data, the FDA has approved 14 ADCs for the treatment of cancer. These ADCs target a range of cancer types including breast cancer, lymphoma, leukemia, and multiple myeloma. Development of ADCs continues, with a considerable number of such drugs currently in clinical trials. The FDA is also reviewing several new ADCs. In reviewing ADCs, the FDA takes a stringent approach, assessing the safety and efficacy of these drugs, while also paying attention to potential off-target effects.
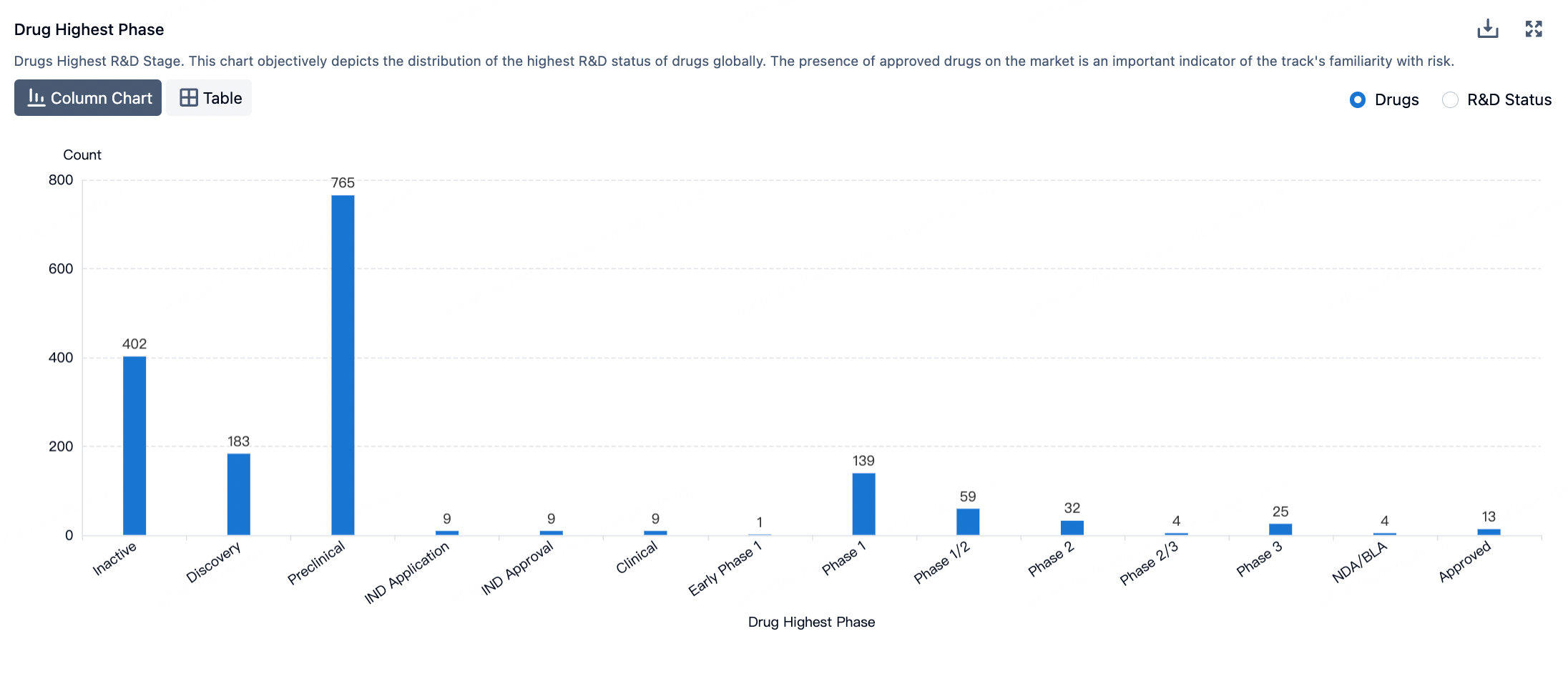
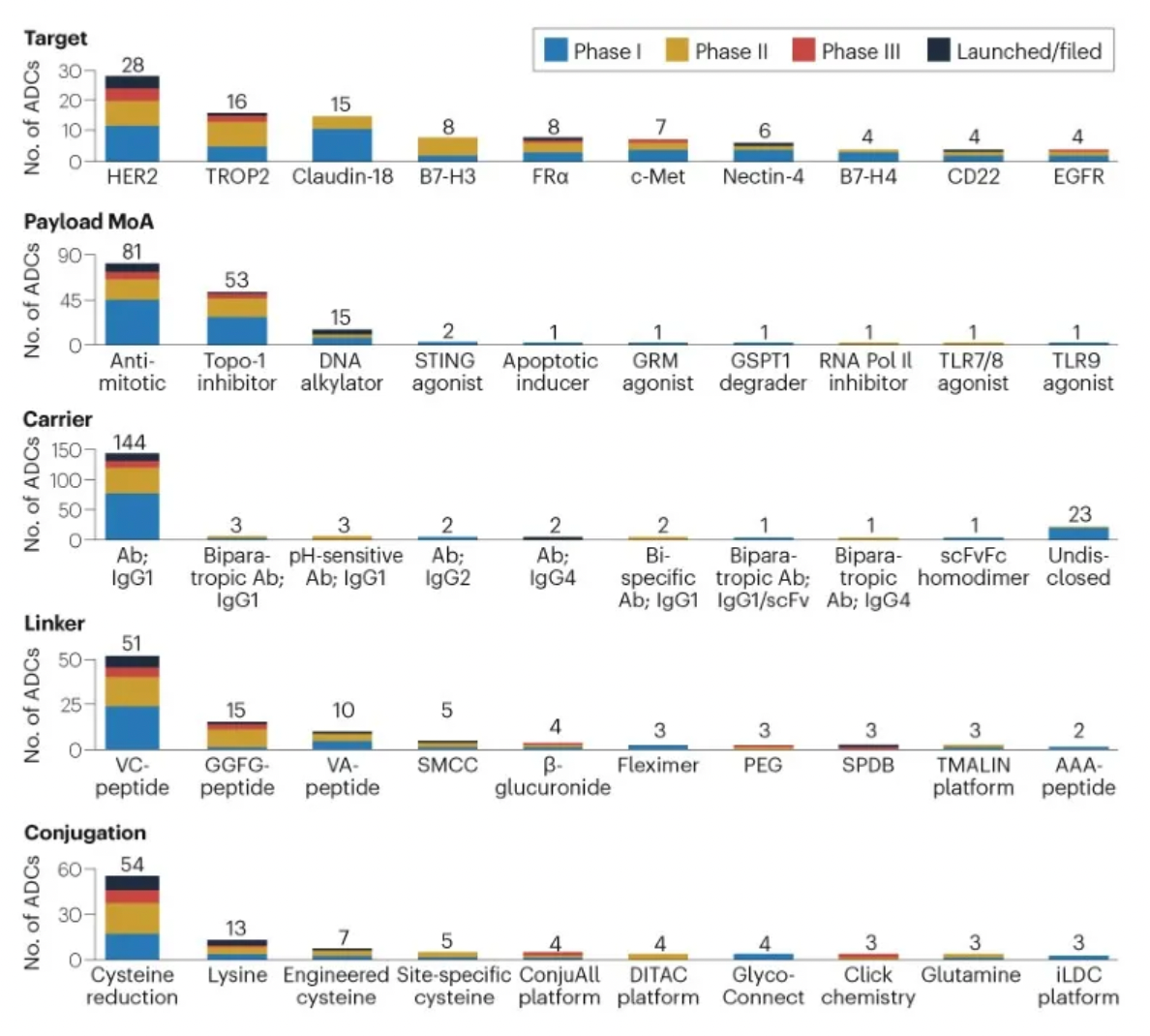
The figure below evaluates the antibody-drug conjugates in clinical development phases, showcasing specific design innovations utilized in clinical assets.
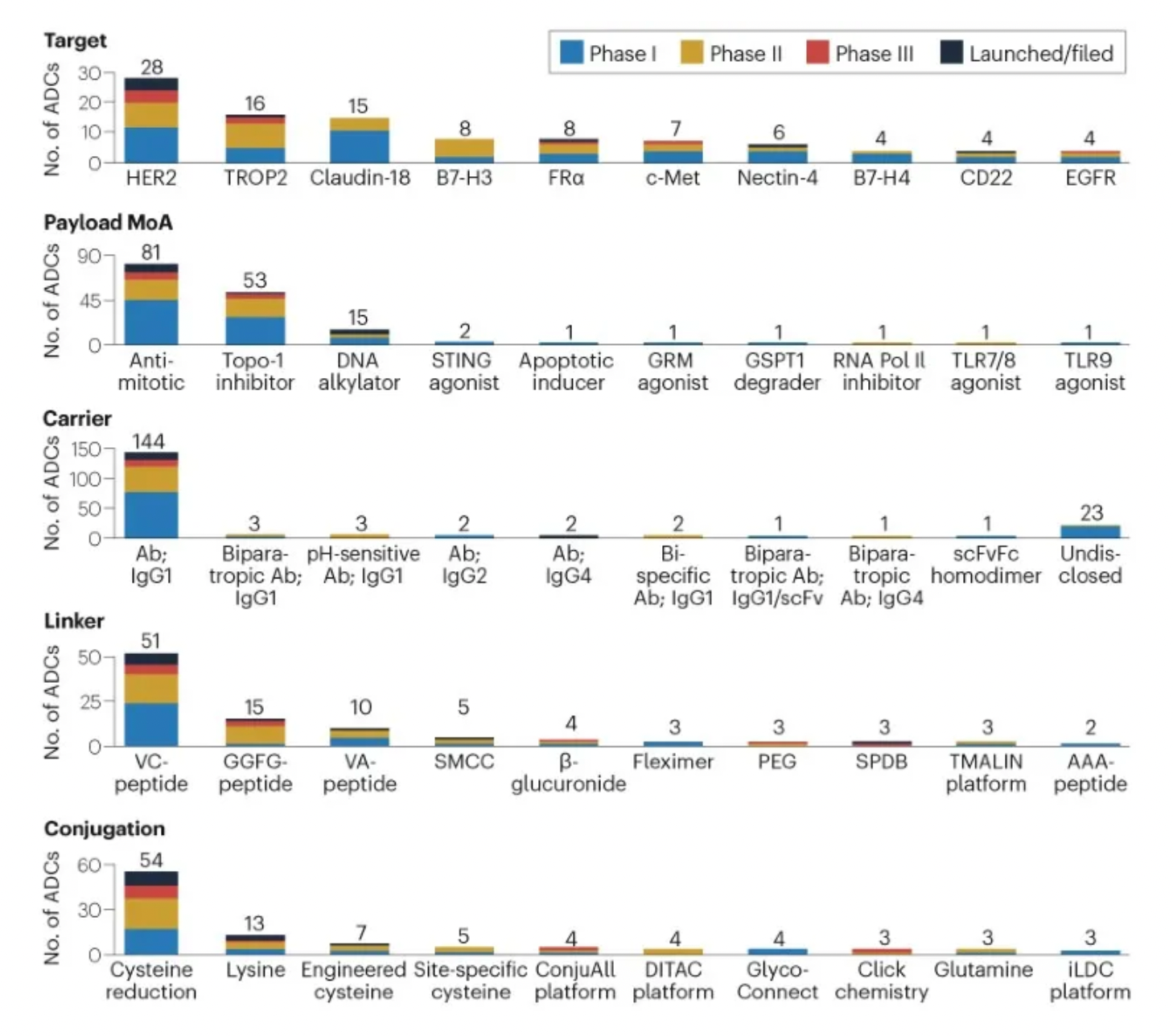
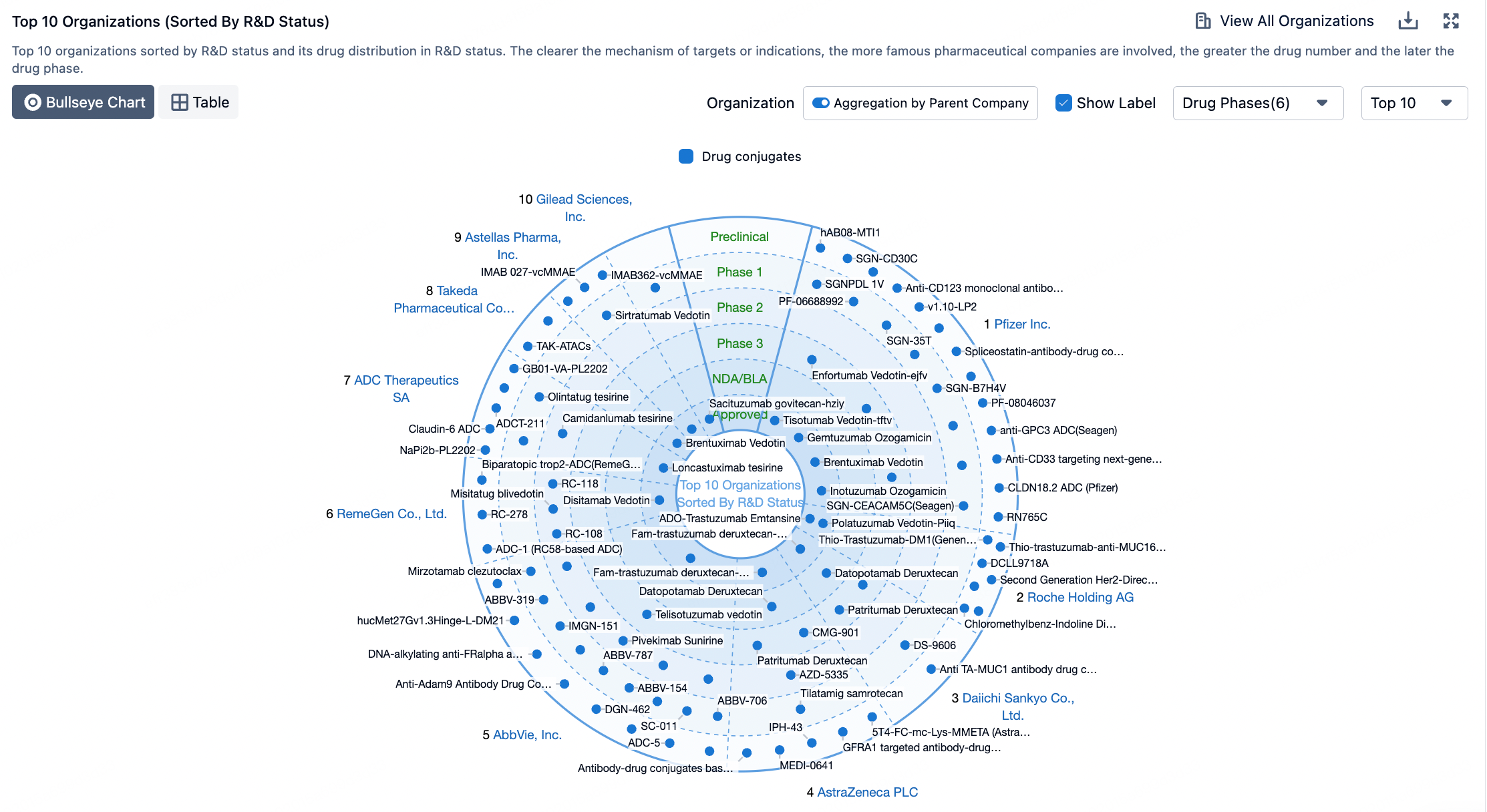
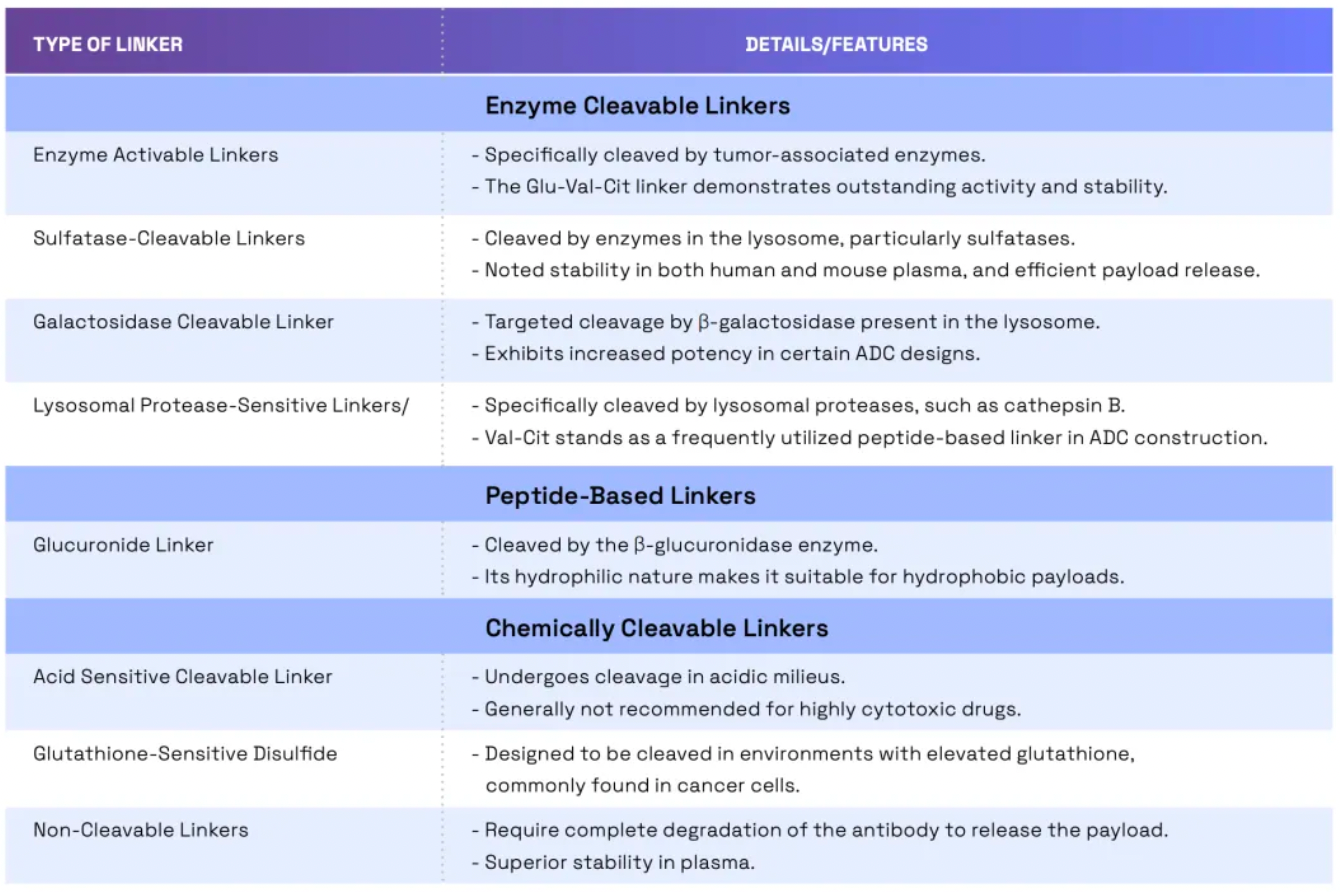
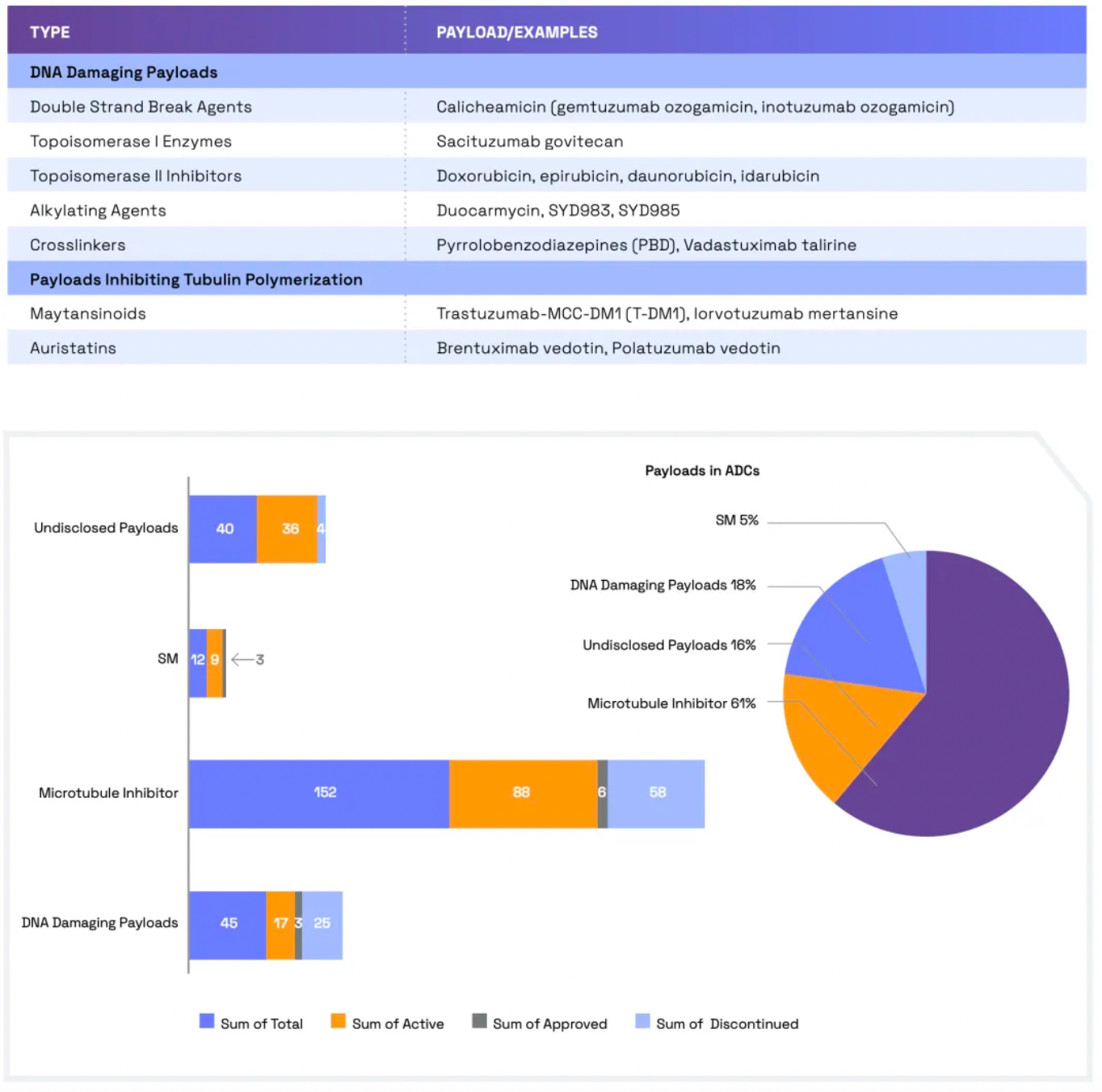
Linkers play a crucial role in ensuring the overall stability of Antibody-Drug Conjugates (ADCs) and the timely release of payloads, having been refined to enhance the therapeutic index. The payload determines the ADC's capability to eliminate cancer cells while minimizing adverse reactions. The figure below presents statistics on linkers and payloads for FDA-approved drugs.
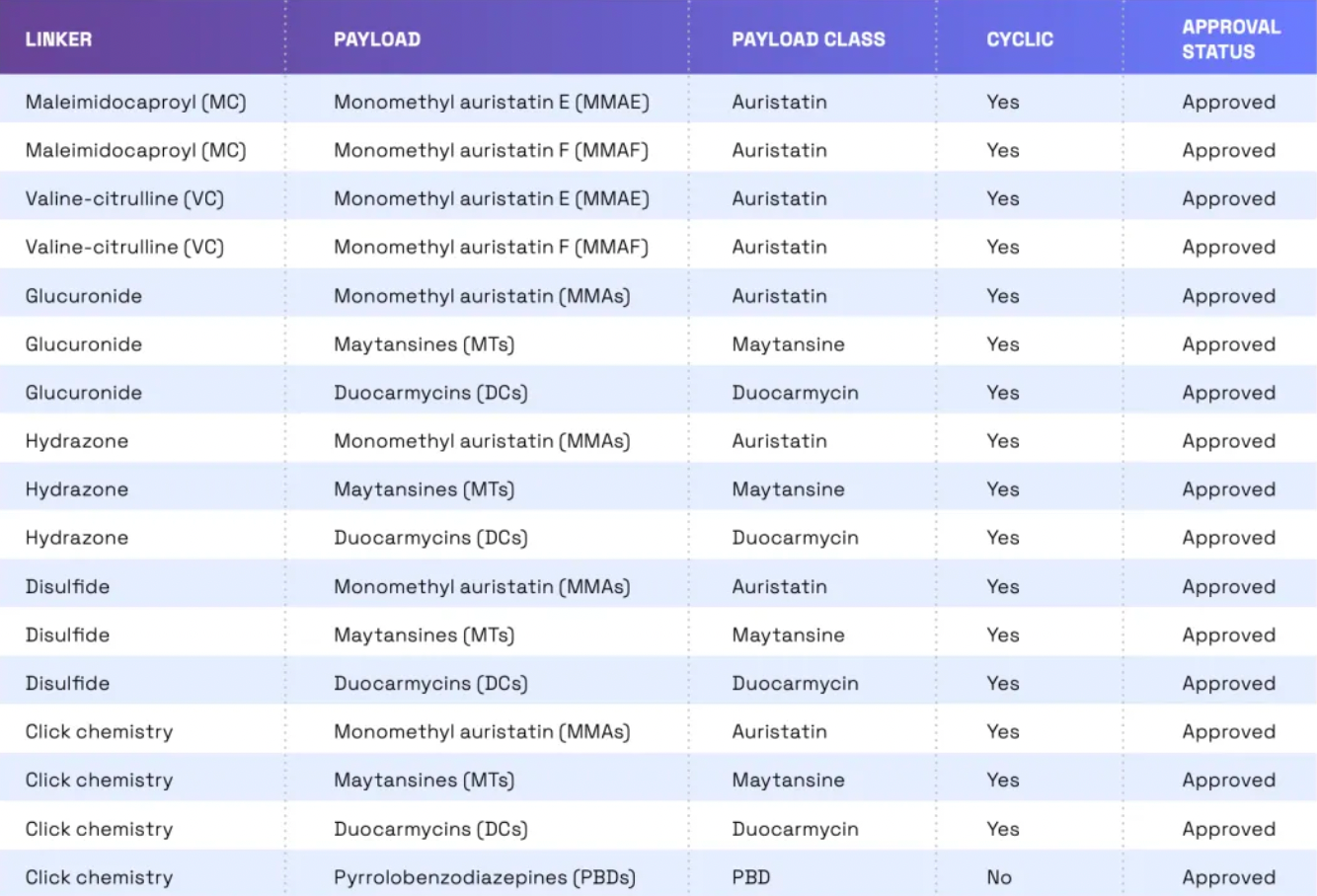
Cleavable linkers utilize differences between the tumor microenvironment and normal physiological conditions to release their drug load. This release can lead to the "bystander effect," where the released drug also affects neighboring cells not directly targeted by the ADC. While this can amplify therapeutic effects, it also poses risks: cleavable linkers are more prone to off-target toxicity. Many clinical trials favor the use of dipeptide, disulfide, and enzyme-cleavable linkers. Enzyme-cleavable linkers, such as those targeting tissue proteases, are designed to be cleaved by enzymes overexpressed in tumor tissues. This specificity adds an extra layer of targeting, further ensuring that the drug is released only at the intended site.
When an ADC binds to its target through antigen-antibody interaction, non-cleavable linkers require the antibody component to degrade before the drug is released. This mechanism can reduce the bystander effect. However, advantages of non-cleavable linkers include greater stability and reduced risk of unexpected side effects. The key challenge in ADC design lies in the antibody's internalization and lysosomal degradation, which are crucial for payload activation. Additionally, payloads linked to polar amino acid linkers require special transport mechanisms to transition from the lysosome to the cytoplasm.
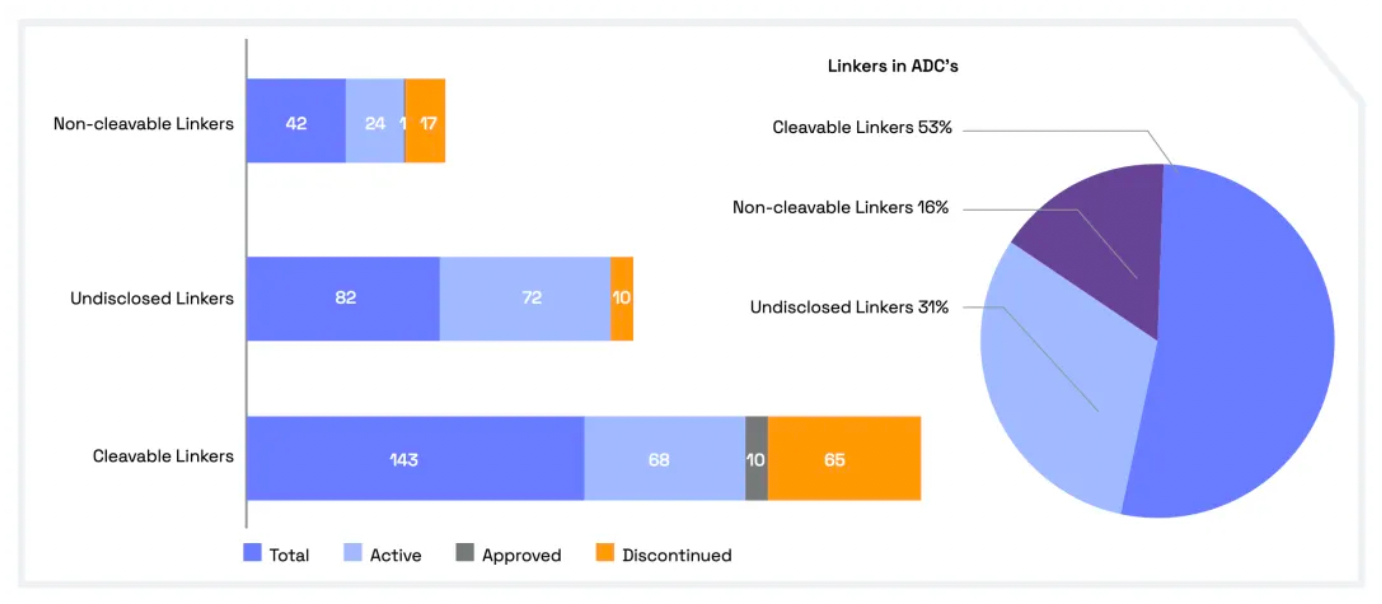
Researchers have been exploring new methods to enhance the efficacy and safety of ADCs (Antibody-Drug Conjugates). By developing novel linkers and potent payloads, researchers aim to create ADCs that more effectively kill cancer cells while exhibiting lower toxicity to healthy cells.
The ideal payload should display high cytotoxicity at low concentrations, enabling effective targeting of tumor cells. The selection of payloads depends on several parameters, including solubility, hydrophilicity, permeability, and modifiability. The design process also needs to consider potential challenges such as aggregation, bystander effects, and stability in the bloodstream.
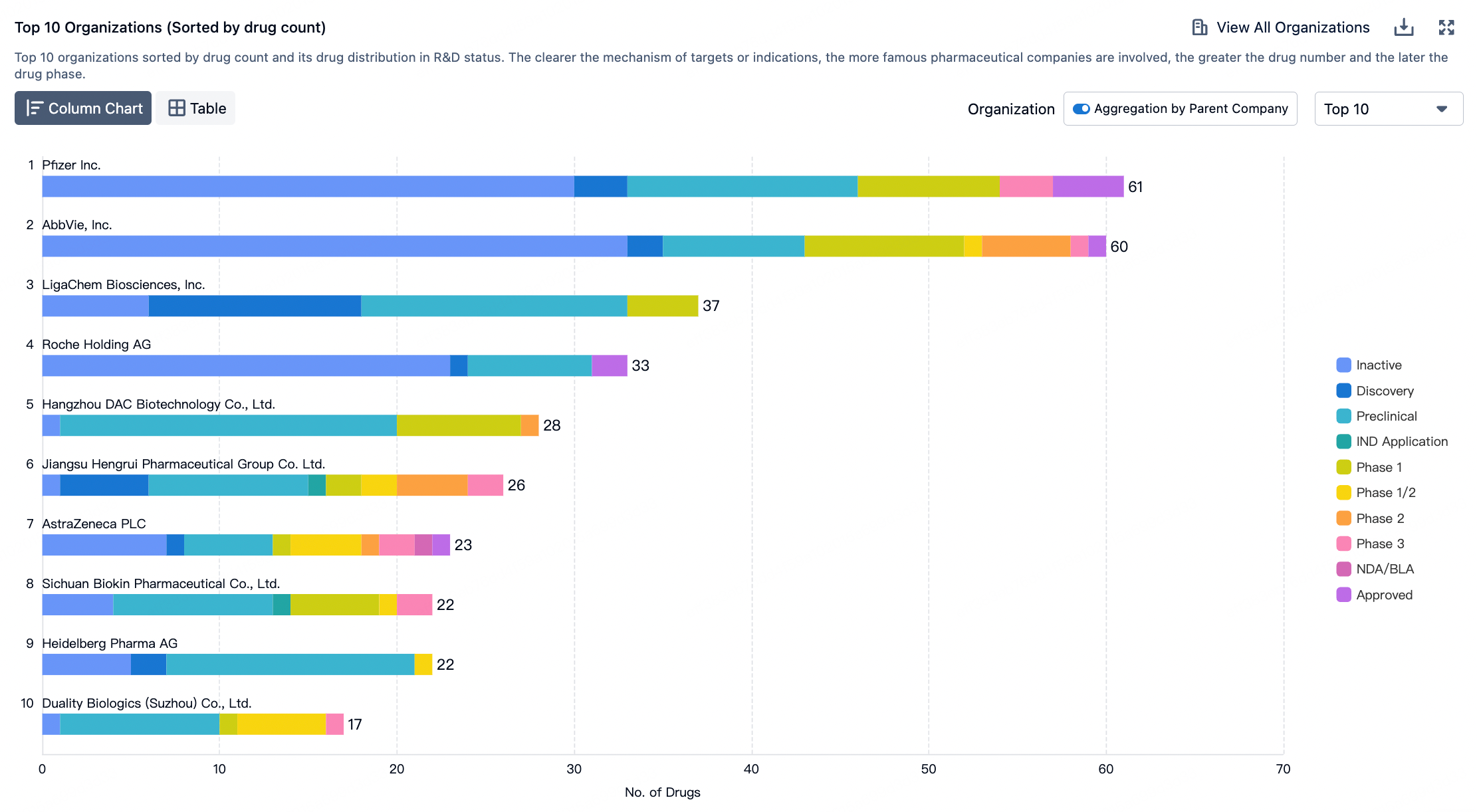
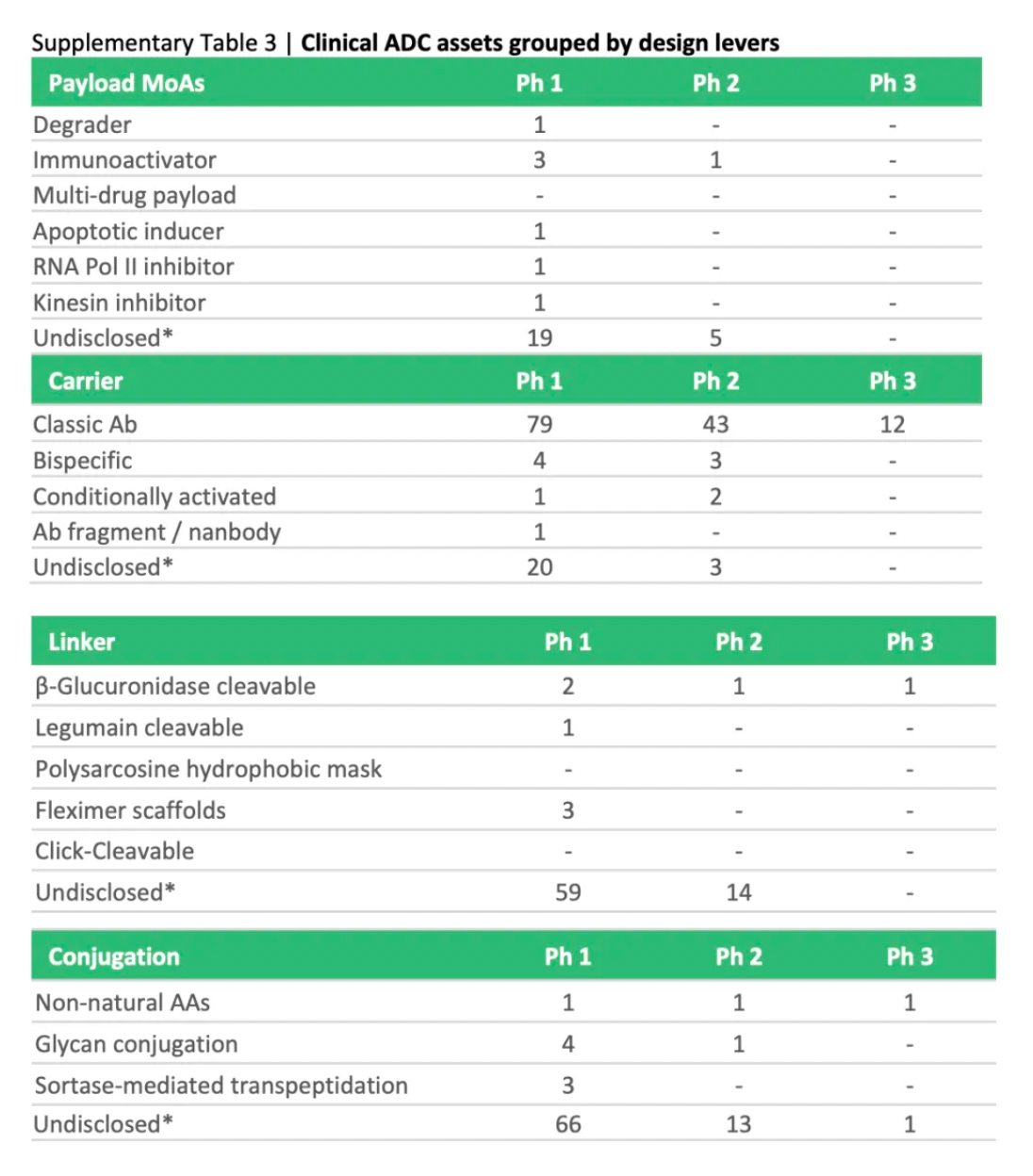
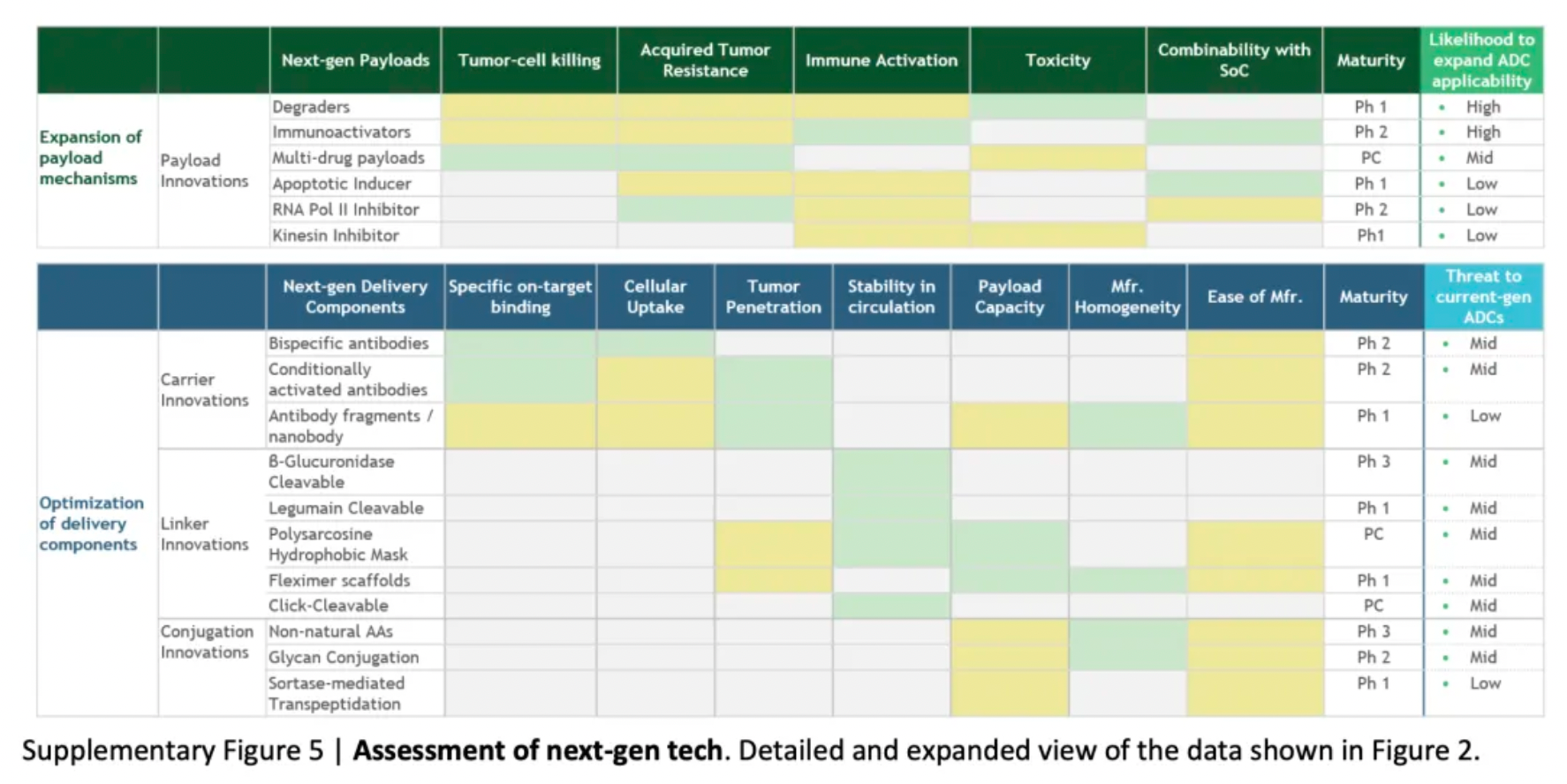
To assess the potential impact of ADC innovations, the diagram below categorizes next-generation technologies and compares them to the characteristics of ADCs that are already on the market. Given their novelty and/or the encouraging preclinical and clinical data, certain designs may be particularly noteworthy.
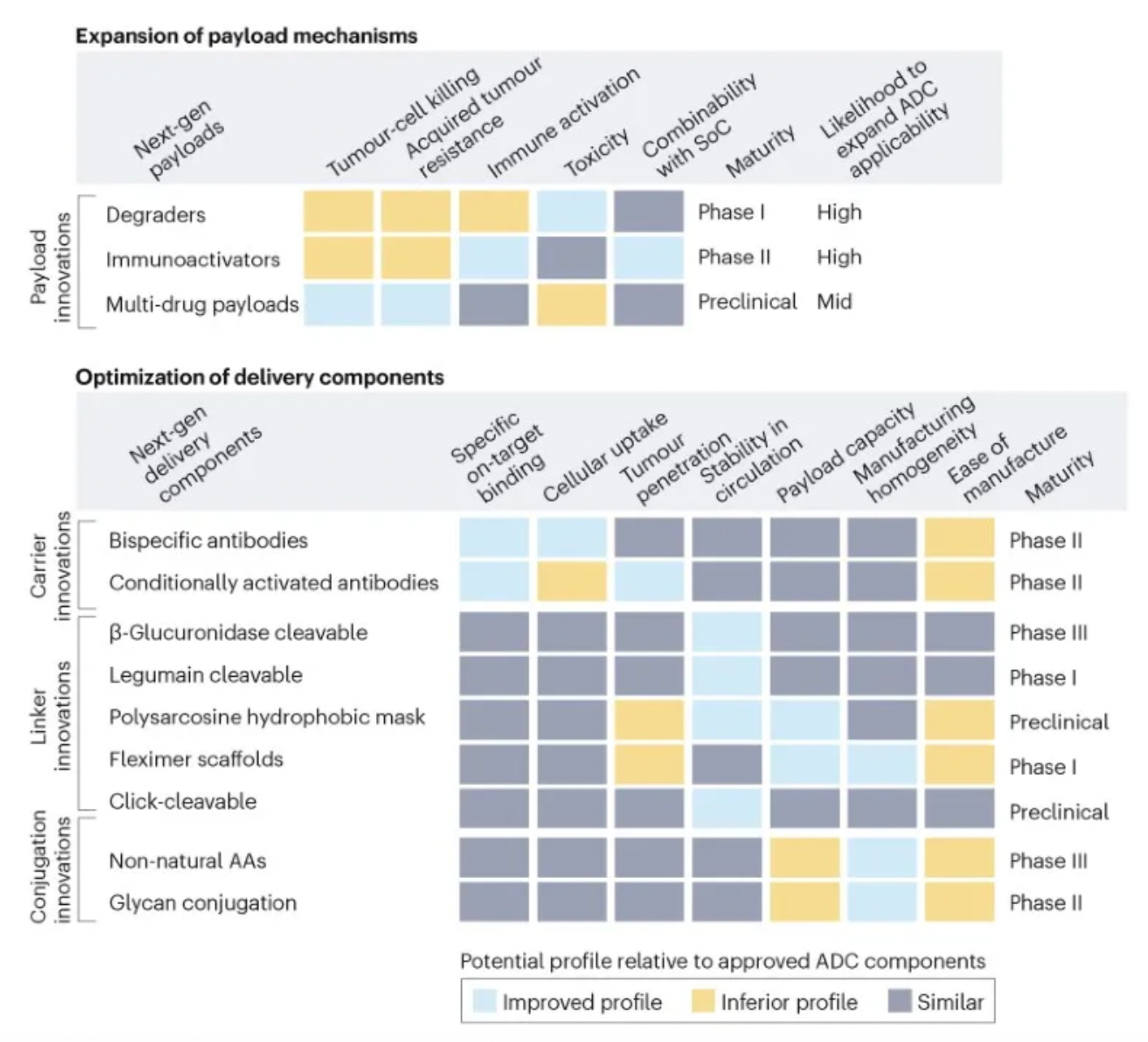
Next-generation payloads: Small molecule degraders as a payload category exhibit significant potential, as they possess high specificity, sub-nanomolar efficacy, and the ability to target an extensive range of intracellular proteins associated with cancer. For instance, Orum Therapeutics' ORM-5029 delivers a selective degrader payload targeting GSPT1, a GTPase overexpressed in multiple cancers including gastric, colorectal, and breast cancer.
Next-generation carriers: Engineering antibodies to have variable antigen-binding affinity can reduce toxicity to non-target tissues and increase tumor-specific exposure. Strategies include masking the Fab domains with a peptide that is cleavable by proteases overexpressed in tumors, and engineering antibodies to optimize pH-sensitive binding characteristics. For example, Mythic Therapeutics’ MYTX-011 is designed to have lower antigen affinity at the pH of endosomes, enhancing payload escape from endosomes.
Next-generation linkers: Emerging linker technologies focus on controlled payload release independent of endogenous enzyme-mediated cleavage. For example, TagWorks’ preclinical ADC TGW101 uses an exogenously administered chemical activator to induce payload release.
Next-generation conjugation: Incorporating non-natural amino acids into antibody carriers facilitates site-specific conjugation through oxime linkages. For example, Ambrx's ARX788 is a HER2-targeted ADC where a non-cleavable PEG linker is attached site-specifically to a non-natural amino acid.
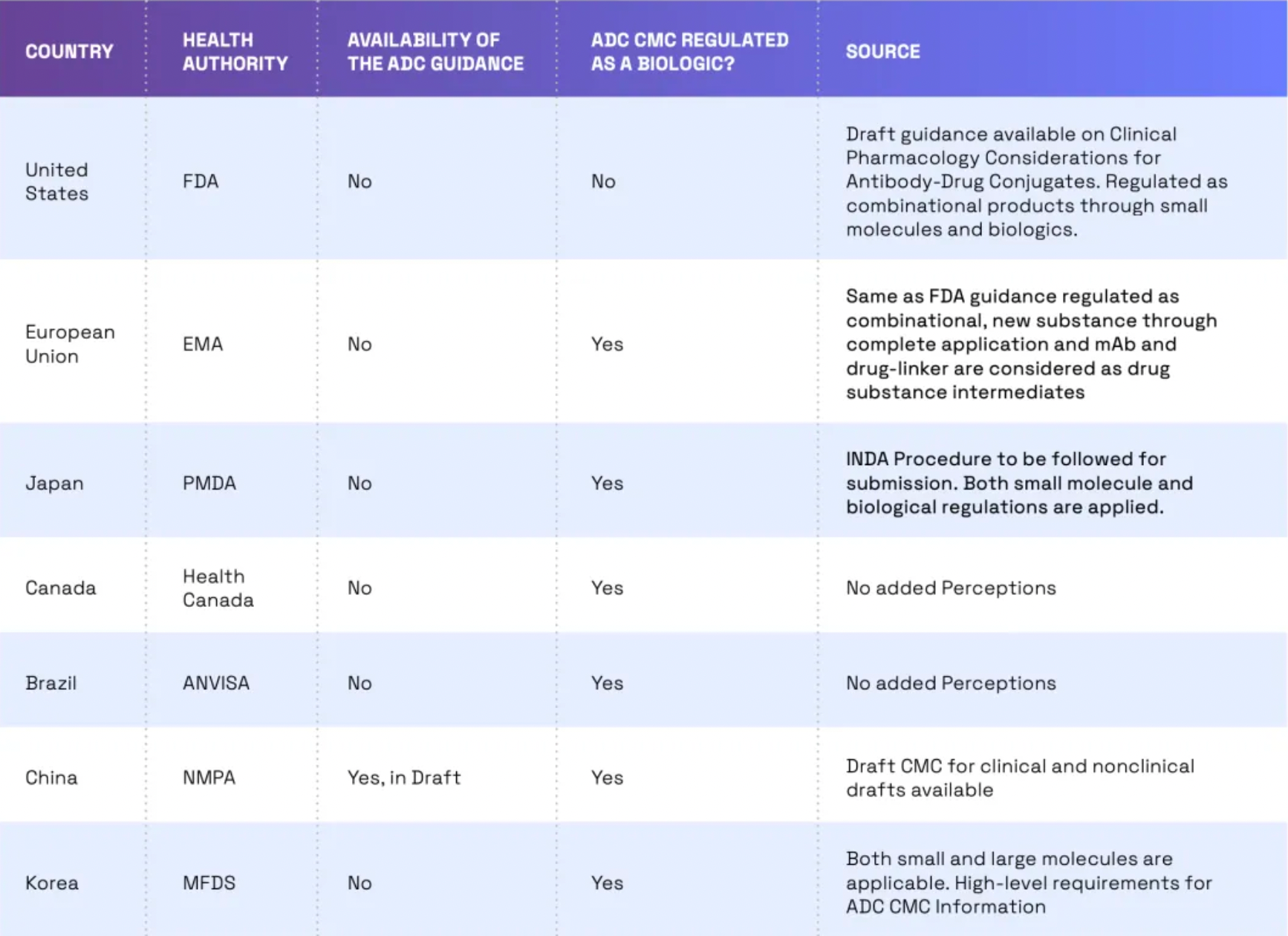
Globally, the trend of regulating ADCs (Antibody-Drug Conjugates) as biologics is growing, yet there are still some differences in regulatory requirements across different countries. For instance, in the United States, ADCs are regulated by the FDA as combination products. This means that both the small molecule drug component and the monoclonal antibody must receive FDA approval before the ADC can be marketed. In the European Union, ADCs are regulated by the European Medicines Agency (EMA) as biologics. In China, ADCs are regulated by the NMPA (National Medical Products Administration) as biologics, and draft guidelines on ADC CMC (Chemistry, Manufacturing, and Controls), clinical, and non-clinical aspects have already been issued.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!