Azitra Announces Patient Screening and Enrollment for Phase 1b ATR-12 Trial in Netherton Syndrome
Azitra, Inc. (NYSE American: AZTR), a precision dermatology firm, has announced the successful screening of the initial patient in their Phase 1b clinical study assessing ATR-12 for Netherton syndrome therapy. The patient is expected to begin treatment before the month's end. This trial aims to recruit roughly 12 adult participants, administering the treatment twice daily over a 14-day period. The primary objectives include evaluating safety and tolerability, while secondary and exploratory goals will focus on determining efficacy signals and biomarkers. Azitra plans to disclose interim safety findings from the Phase 1b trial in early 2025, with complete results projected for release in the latter half of 2025.
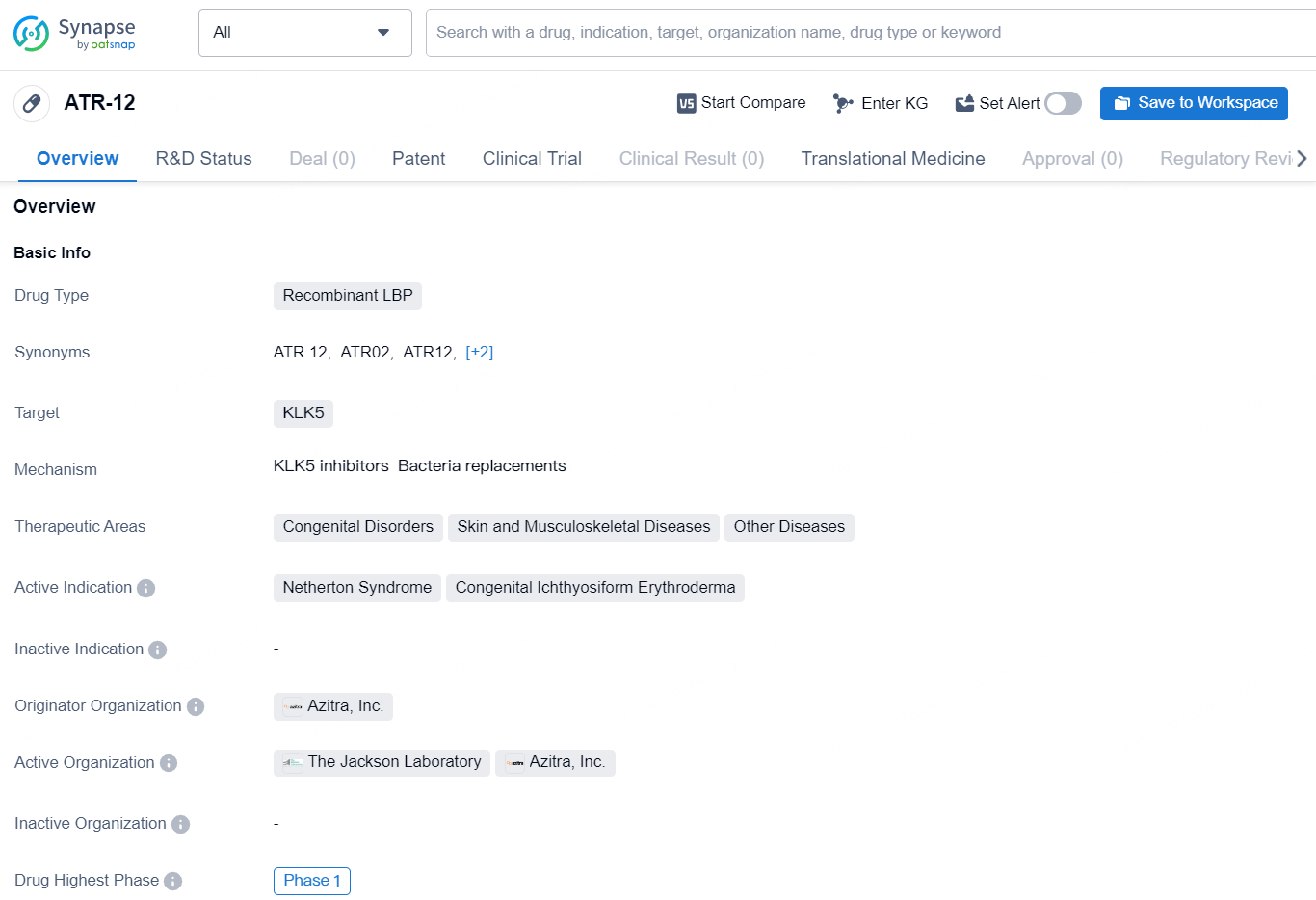
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ATR-12, Azitra’s flagship candidate, is an innovative strain of Staphylococcus epidermidis designed to produce therapeutic amounts of an active LEKTI protein subunit for the treatment of Netherton syndrome, a severe chronic genetic skin disorder.
"The initiation of our Phase 1b trial for ATR-12 is a crucial step forward for Azitra, and more importantly, for patients with Netherton syndrome who have very limited treatment options," stated Travis Whitfill, founder and COO of Azitra. "Our latest preclinical results show that ATR-12 can effectively deliver the active LEKTI subunit to the skin and target the fundamental causes of Netherton syndrome."
Azitra’s interim CMO, Dr. Mary Spellman, commented, "We are excited to commence this clinical trial involving Netherton syndrome patients. These individuals experience a significant reduction in quality of life due to this debilitating condition. This trial will provide insights for future pediatric treatments and longer-term therapy options for Netherton syndrome."
The Phase 1b trial (NCT06137157) is a multicenter, randomized, double-blind, vehicle-controlled study involving around 12 adult patients with Netherton syndrome. Participants will receive 109 CFU / g of ATR-12 or a vehicle control on opposite sides of the body twice a day for 14 days. The main goal is to evaluate the safety and tolerability of topical ATR-12. Secondary goals include determining efficacy signals such as investigator and patient global assessments and skin pharmacokinetics of the LEKTI subunit. Additional exploratory objectives include assessing pharmacodynamic parameters, biomarkers, anti-LEKTI response, and cytokine responses.
The trial is supported by robust preclinical data presented at the 2024 Annual Meeting of the American Society of Gene and Cell Therapy (ASGCT). Key findings included:
Topical application of ATR-12 in preclinical models reduced IL-36γ by 93% compared to skin extracts induced to overexpress IL-36γ.
ATR-12 significantly lowered protease activity in skin samples compared to a Netherton syndrome model (p<0.01).
ATR-12 generated higher levels of the LEKTI subunit than the direct application of LEKTI protein alone (6.0 µg vs. 2.3 µg, p<0.01) after 24 hours and achieved better skin penetration of the LEKTI protein.
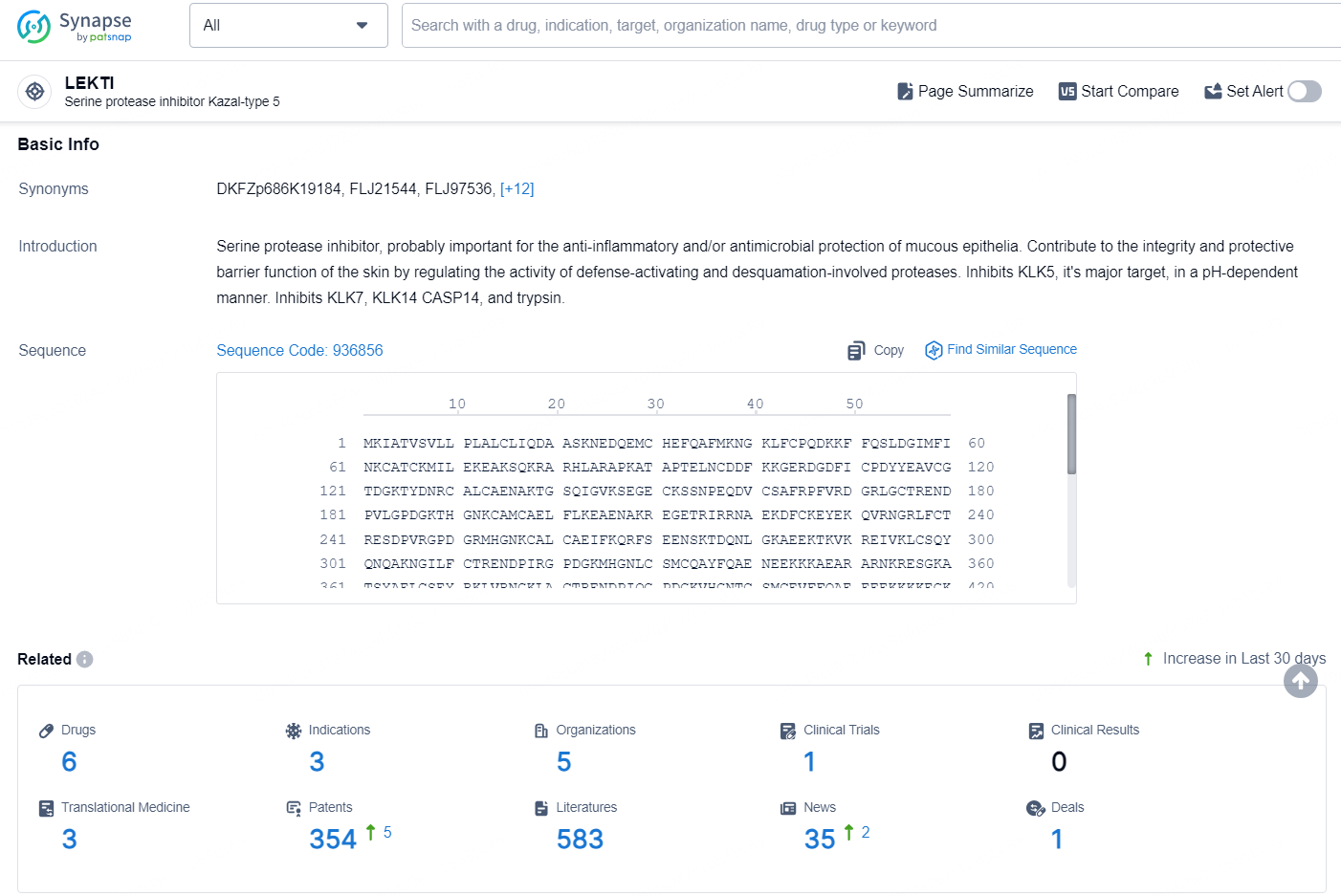
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 23, 2024, there are 6 investigational drugs for the LEKTI targets, including 3 indications, 5 R&D institutions involved, with related clinical trial reaching 1, and as many as 354 patents.
ATR-12 (also referred to as ATR12-351) is a modified strain of S. epidermidis designed to produce an active subunit of the human lympho-epithelial Kazal-type related inhibitor (LEKTI) protein, which is deficient in individuals with Netherton syndrome. This chronic and sometimes lethal skin disorder is estimated to affect about one to nine people per 100,000. ATR-12 aims to supply the absent LEKTI protein through topical application to Netherton syndrome sufferers. Azitra currently has an active IND for a Phase 1b clinical trial involving adult patients (NCT06137157). The company has established clinical sites and identified patients with Netherton syndrome for inclusion in its 12-patient Phase 1b clinical trial, which will evaluate safety, tolerability, and effectiveness metrics. Azitra anticipates releasing initial safety data before the end of the year.