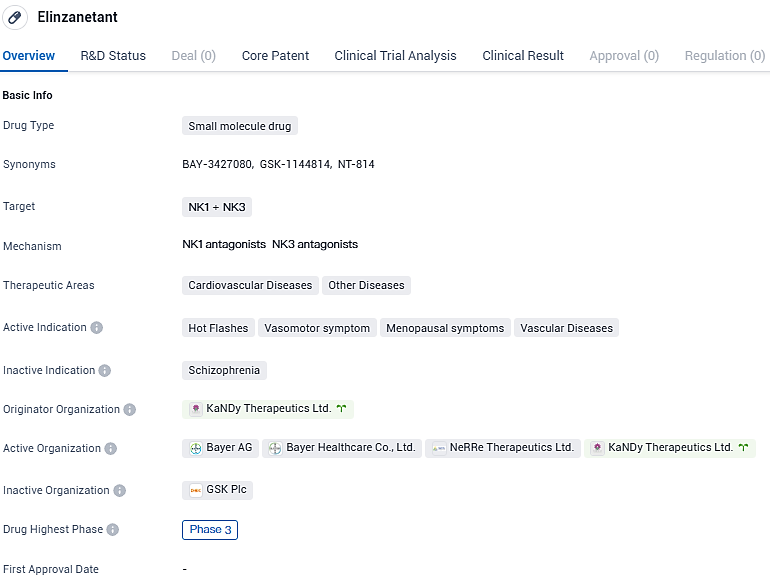
Bayer's new drug elinzanetant successfully achieves main and critical additional goals in essential OASIS 1 and 2 Phase III trials
Bayer has reported encouraging preliminary outcomes from the critical Phase III trials, OASIS 1 and 2, examining the performance and security of the test compound elinzanetant against a placebo. In both trials, elinzanetant achieved all four principal goals, showing a notable statistical decrease in occurrence and intensity of moderate to intense vasomotor symptoms between the start of the study and weeks 4 and 12 when measured against a placebo.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Both research projects additionally met all three crucial secondary outcomes, demonstrating a marked and statistically notable decline in the occurrence of vasomotor symptoms (VMS) starting from the first week, along with substantial enhancements in sleep issues and the menopause-specific quality of life when juxtaposed with the placebo group. The tolerability profile identified within the OASIS 1 and 2 trials aligns with earlier released findings1,2 about elinzanetant.
Dr. Christian Rommel, a part of Bayer AG's Pharmaceutical Division Executive Committee and the chief of Global Research and Development, expressed enthusiasm for the encouraging outcomes of these landmark Phase III trials for elinzanetant, further asserting its promise as a novel non-hormonal therapy in managing menopause symptoms.
Elinzanetant stands out as the pioneering compound to target both neurokinin-1,3 receptors and is currently in the advanced phase of testing for addressing moderate to intense VMS tied to menopause with a once-daily oral dose.
JoAnn Pinkerton, M.D., a Midlife Health Professor and Director at UVA Health, remarks on the intense disruption caused by menopausal symptoms like hot flashes and sleep difficulties which broadly affect overall health and lifestyle quality. She notes that many women endure without seeking treatment, stressing the need for ongoing investigation into interventions that fulfill these women's requirements. Dr. Pinkerton is also anticipating the comprehensive disclosure of the study findings.
OASIS 1 and 2 constitute the initial Phase III trials part of the overarching OASIS clinical research initiative to have their results publicized, with detailed findings slated for presentation at imminent scientific meetings. The outcomes from a third pivotal Phase III evaluation, OASIS 3, are awaited shortly. Bayer intends to petition for marketing approval from regulatory agencies based on data from the OASIS 1, 2, and 3 trials, targeting the treatment for moderate to severe menopause-related VMS.
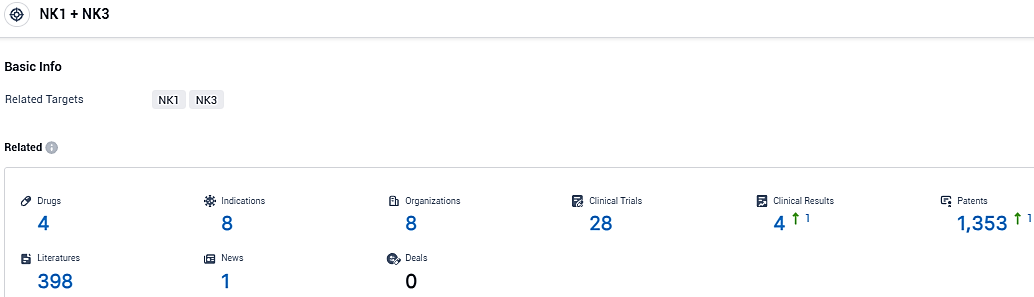
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 12, 2024, there are 4 investigational drugs for the NK1 and NK3 tagets, including 8 indications, 8 R&D institutions involved, with related clinical trials reaching 28, and as many as 1353 patents.
Elinzanetant is a first dual neurokinin-1,3 receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe VMS associated with menopause, administered orally once daily. Elinzanetant may address moderate to severe VMS by modulating a group of estrogen sensitive neurons in the hypothalamus region of the brain which, with the decrease of estrogen, become hypertrophic and lead to a hyperactivation of the thermoregulatory pathway, consequently disrupting body heat control mechanisms resulting in VMS.