Exploring the Latest c-MET ADC Deal by MediLink Therapeutics: A Guide to Rapidly Accessing Transaction Insights
On January 2, 2024, MediLink Therapeutics announced that it has entered into a global collaboration and license agreement with Roche. The parties will collaborate on the development of YL211, a next-generation antibody-coupled drug (ADC) candidate targeting mesenchymal epidermal transition factor (c-MET), for the treatment of solid tumors. Roche will have exclusive rights to develop, manufacture and commercialize YL211 globally; YL211 will be promoted to Phase I clinical trials by YL211 in collaboration with Roche's China Innovation Center (CICoR), with Roche being responsible for further development and commercialization worldwide. Under the terms of the agreement, Roche will make an initial down payment and near-term milestone payments of $50 million to E-Link Bio, plus nearly $1 billion in potential milestone payments for development, registration and commercialization, as well as future graded royalties based on annual global net sales.
About YL211
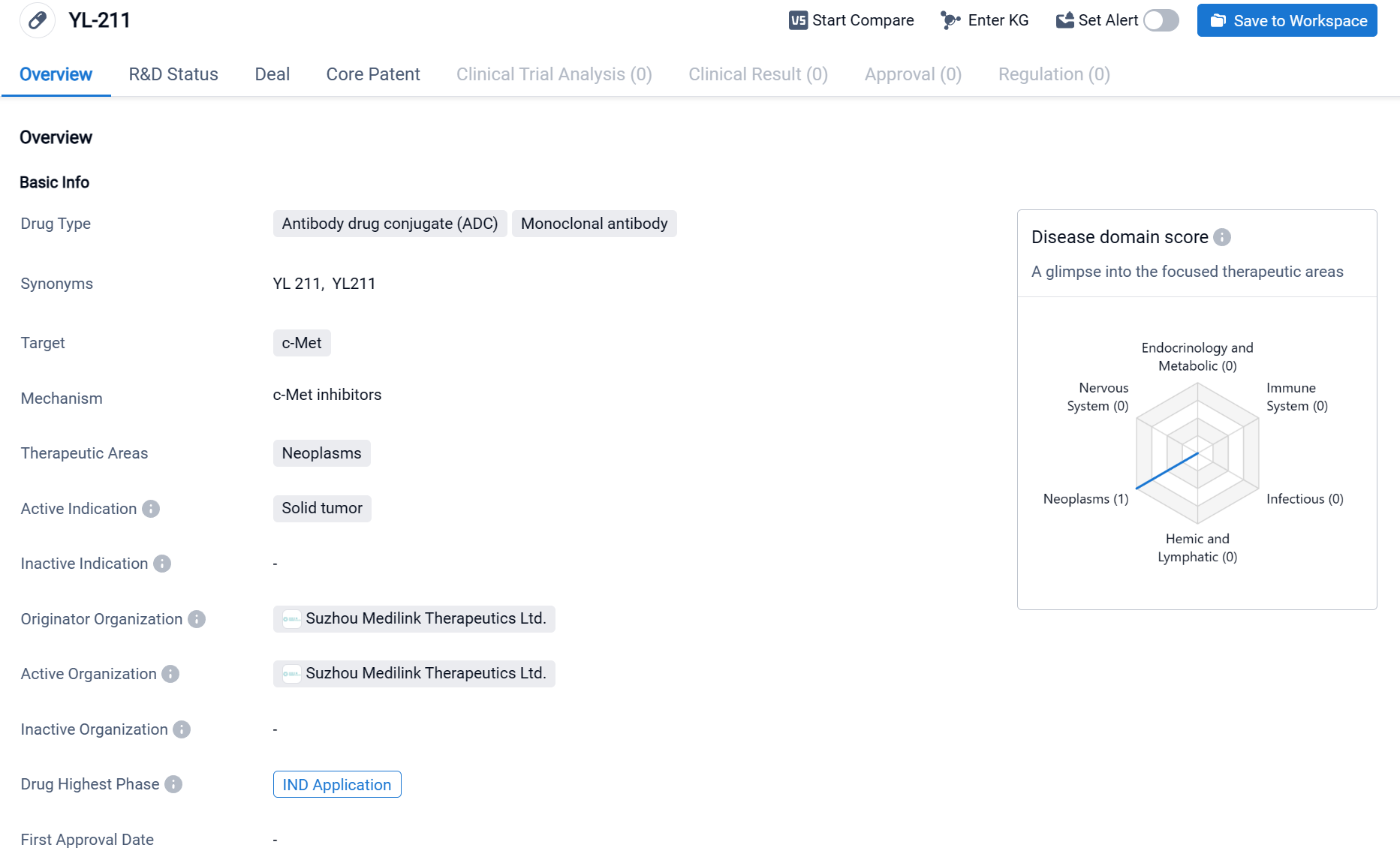
YL-211 is an antibody drug conjugate (ADC) and monoclonal antibody that targets c-Met. It is being developed by Suzhou Medilink Therapeutics Ltd. for the treatment of neoplasms, specifically solid tumors. Currently, YL-211 is in the highest phase of development, which is the IND application phase, both globally and in China. ADCs are a type of targeted therapy that combines the specificity of monoclonal antibodies with the cytotoxic effects of chemotherapy drugs. By attaching a cytotoxic drug to a monoclonal antibody, ADCs can specifically deliver the drug to cancer cells that express the target protein, in this case, c-Met. This targeted approach aims to minimize the side effects associated with traditional chemotherapy while maximizing the therapeutic effect. Click the image below to directly embark on the exploration journey with the YL211!
YL211 is currently at the IND application stage. In various preclinical tumor models and safety evaluation experiments, YL211 has shown promising efficacy and safety potential.
About Medilink Therapeutics
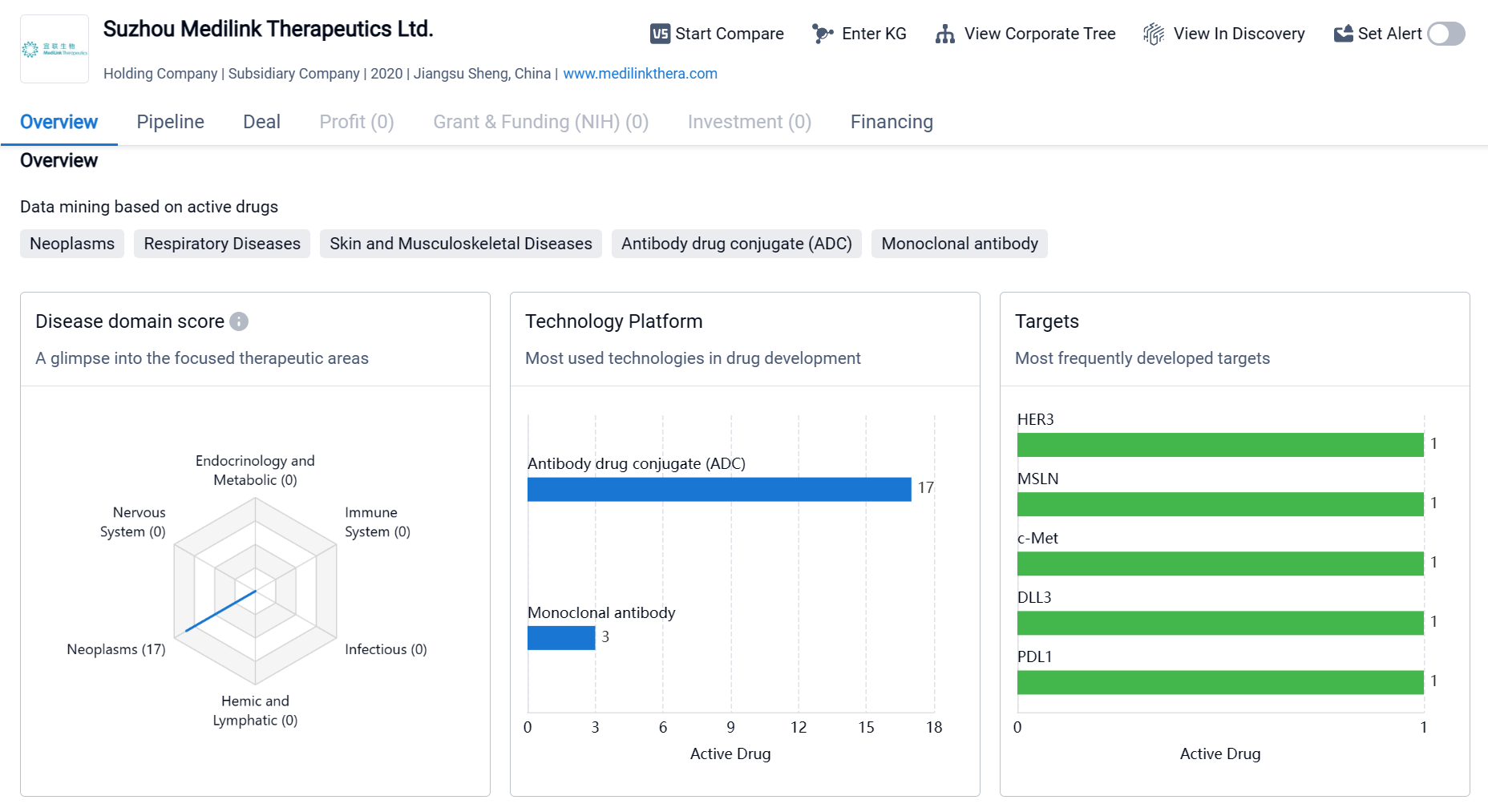
Suzhou Medilink Therapeutics Ltd. is a relatively new biomedicine organization that has been actively involved in drug development since its establishment in 2020. The organization has focused on various therapeutic areas, with a particular emphasis on neoplasms. It has also targeted specific molecules and proteins associated with diseases, indicating a diverse approach to drug development. While the organization has several drugs in the pipeline, most of them are in the early stages of development, with only a few reaching the clinical trial phase.
Focusing on the development of innovative coupling drugs, Medilink Therapeutics has developed the latest generation of Tumor Microenvironment Activable LINker-payload (TMALIN®) novel antibody-coupled drug platform technology with its own intellectual property rights, which can achieve high DAR value homogeneity and stability of the coupling at the same time, further improve the therapeutic window of the ADC drugs and enhance the therapeutic effect of ADC drugs in solid tumors.
How to get the latest progress on drug deals?
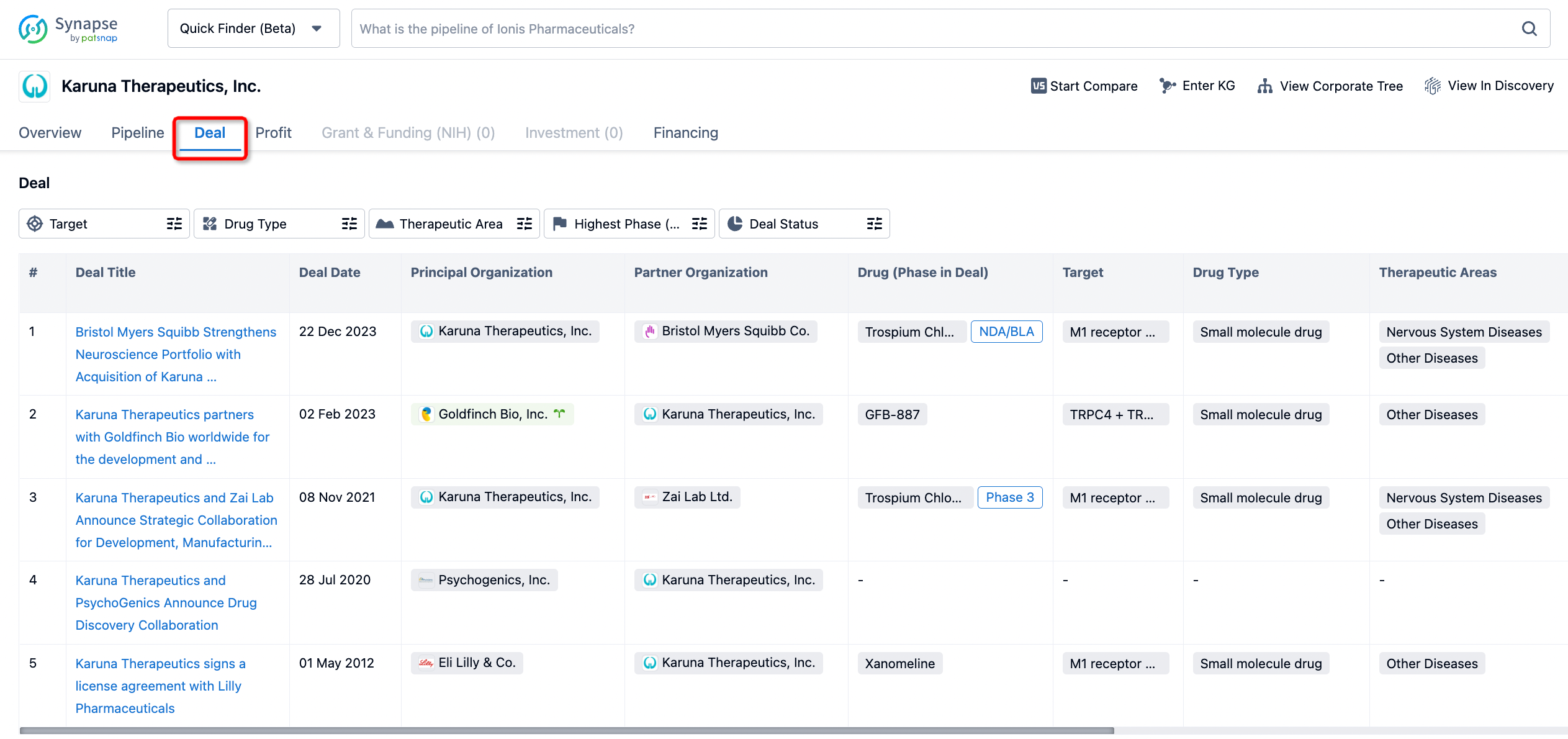
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
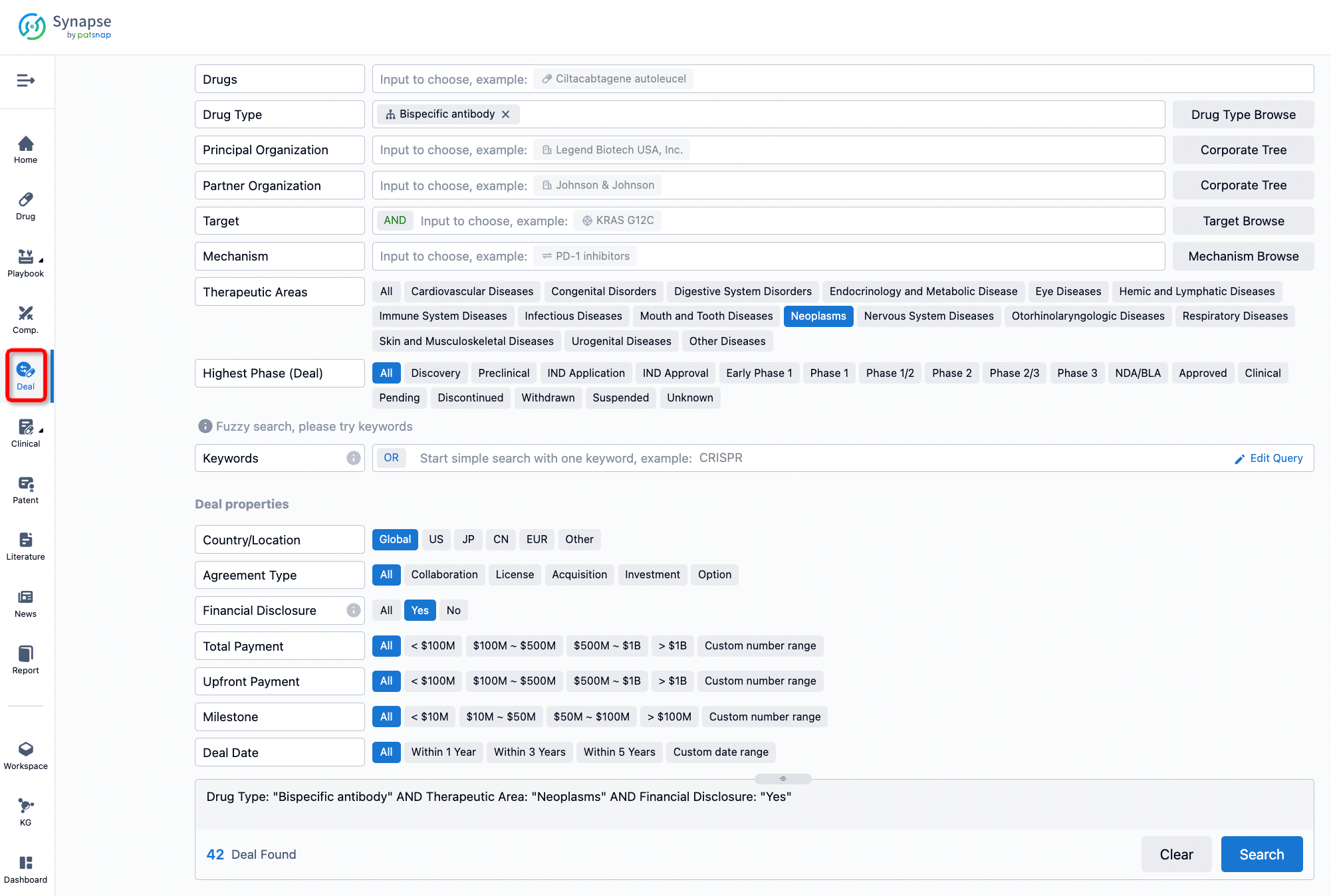
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
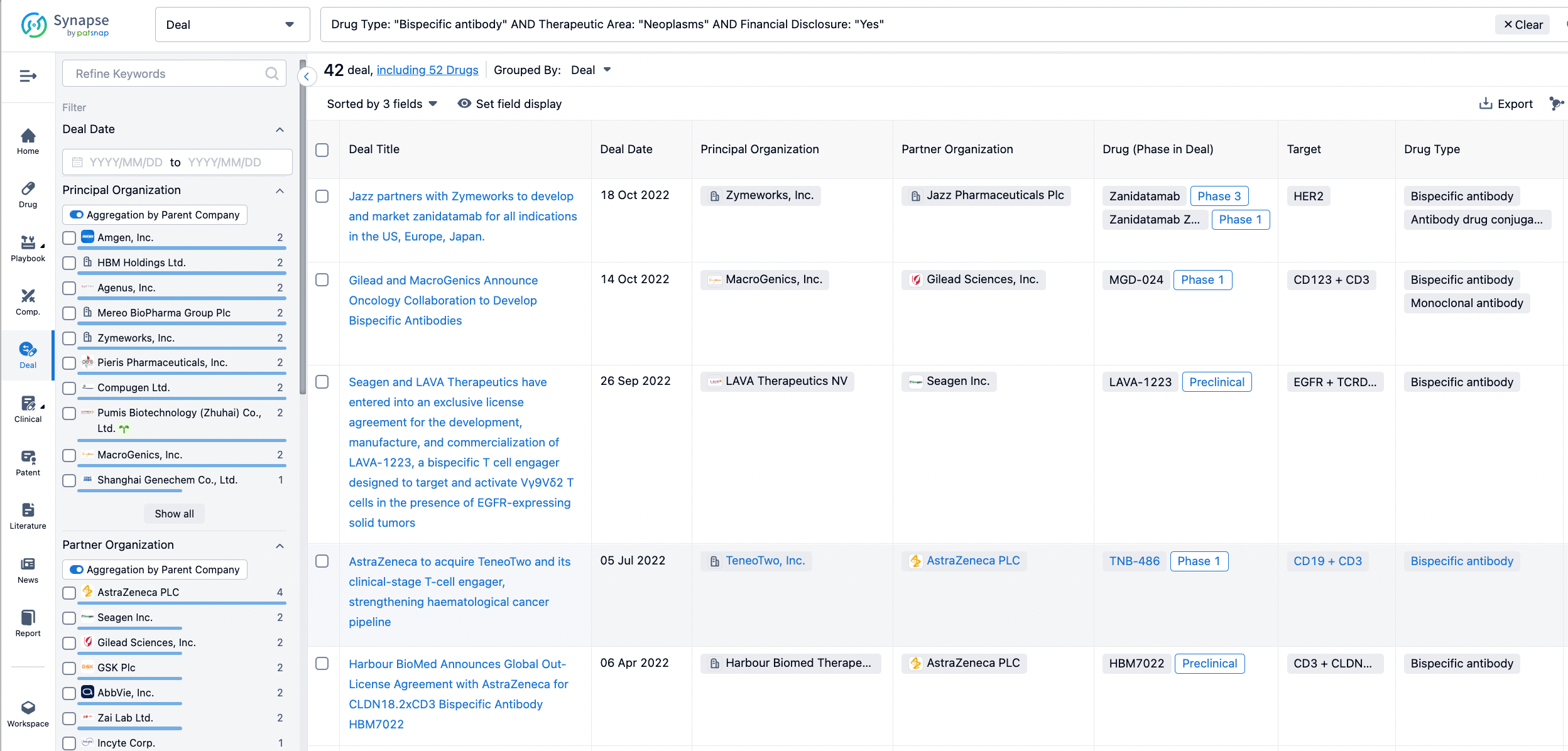
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
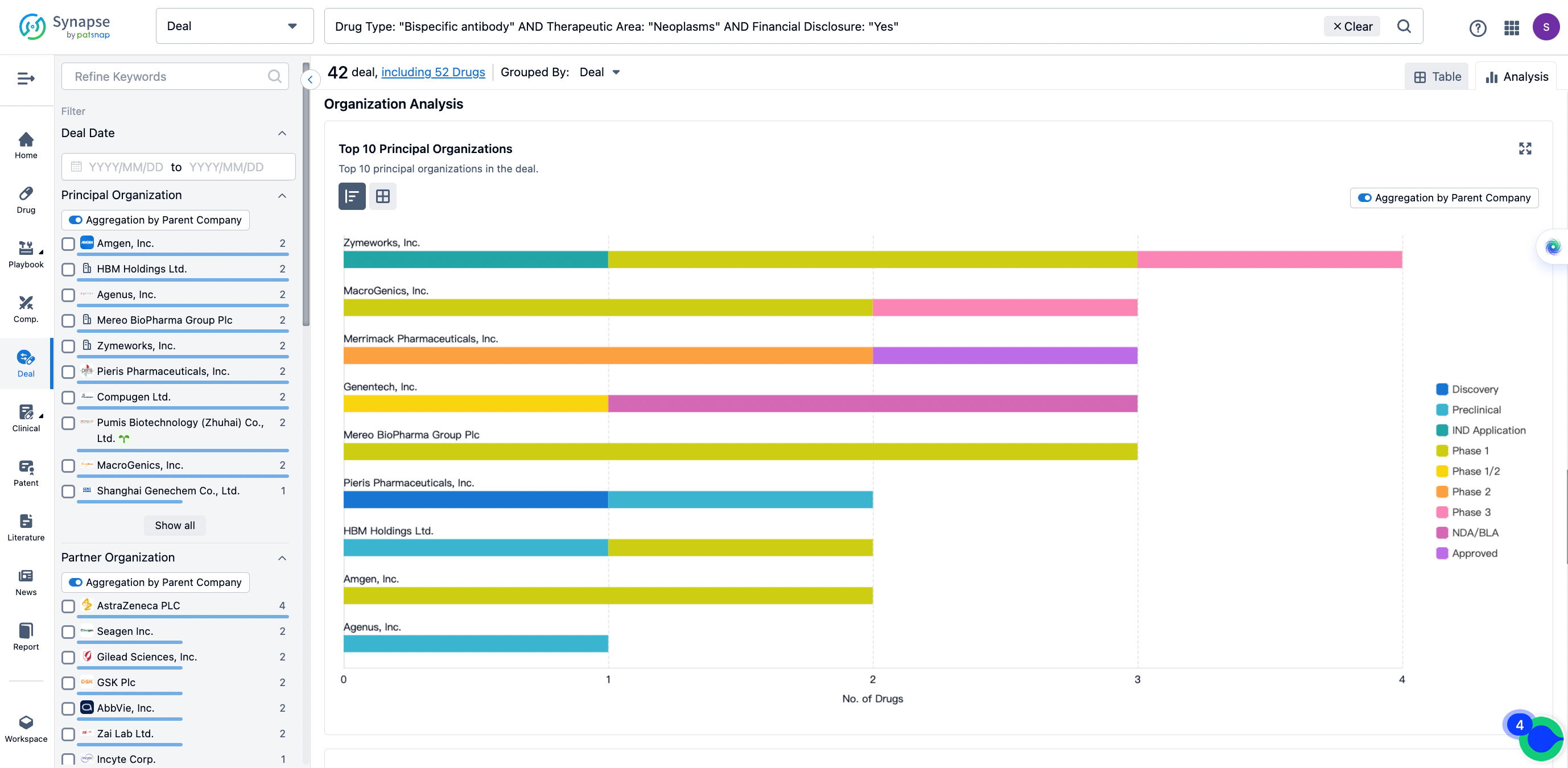
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
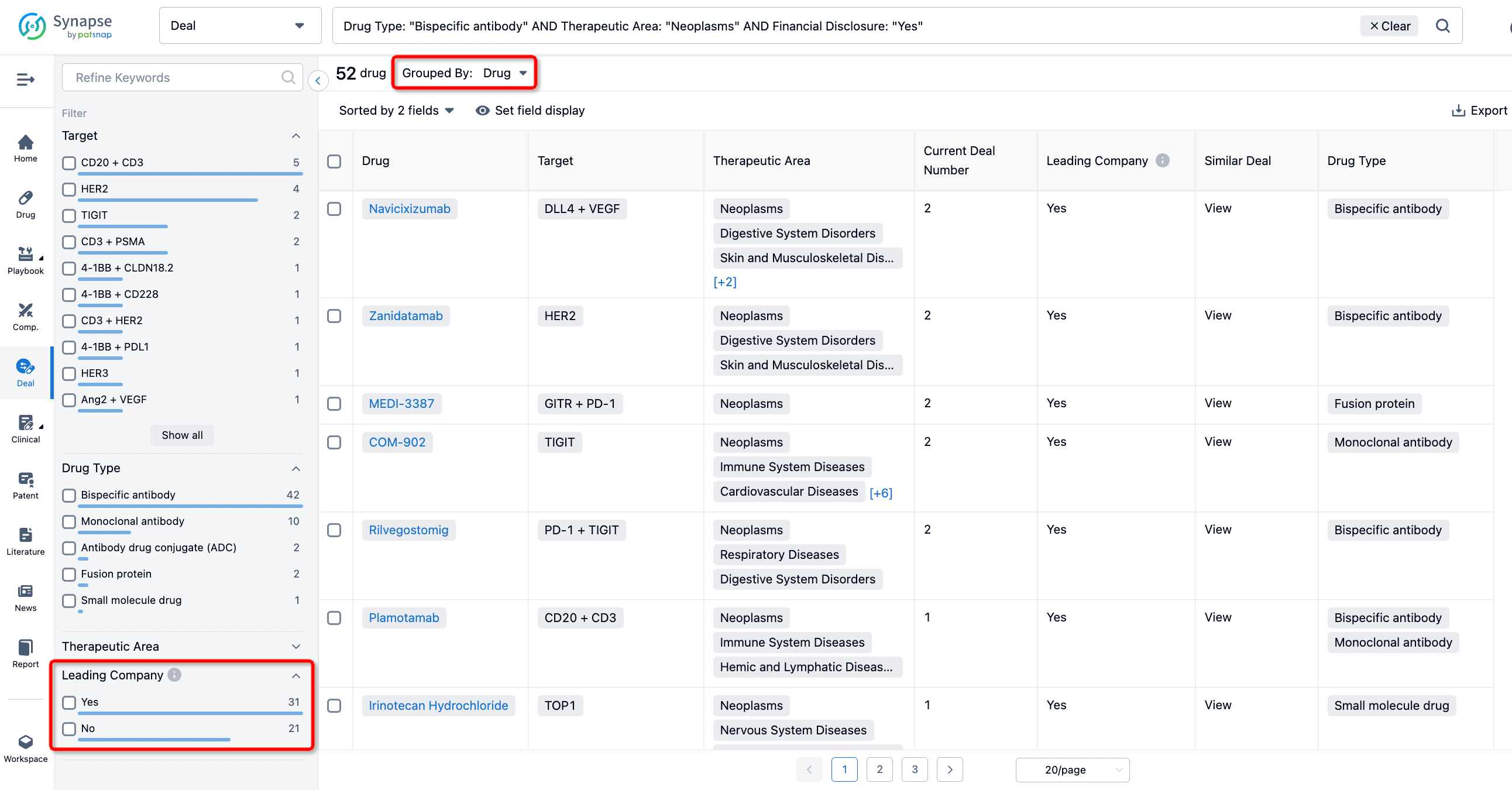
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
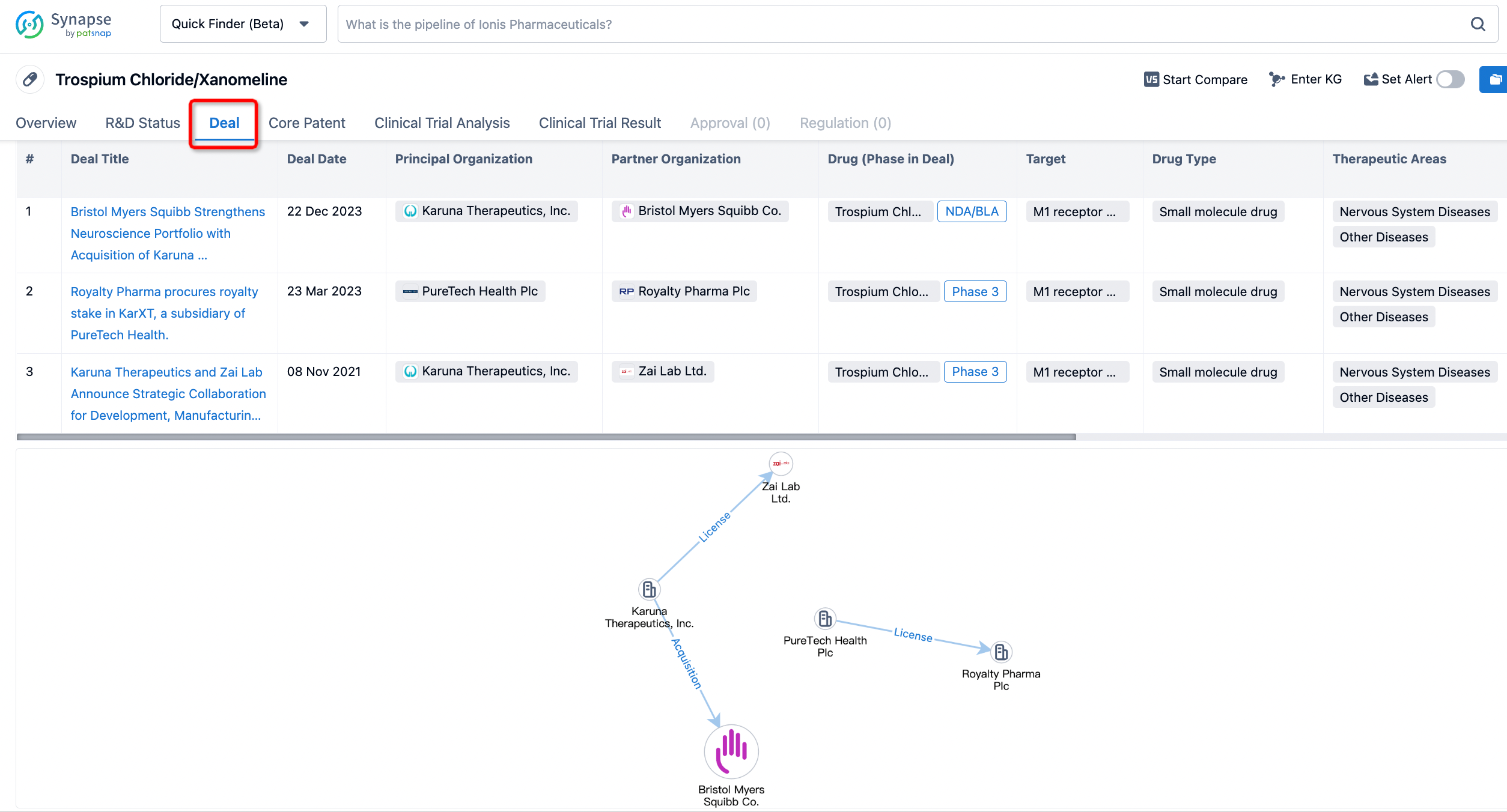
Click on the image below to embark on a brand new journey of drug discovery!