OKYO Pharma Corp. reports that OK-101 hit key efficacy goals in its Phase 2 trials, effectively treating dry eye syndrome
OKYO Pharma Limited, an enterprise at the clinical development phase specializing in the creation of cutting-edge treatments, has announced encouraging findings pertaining to the safety and effectiveness of its product from a Phase 2 study. This research is a scientifically randomized, double-blind, and placebo-managed trial aiming to determine the tolerability and potential benefits of OK-101 eye drops in individuals afflicted with inflammatory dry eye syndrome. As a condition within a market worth several billion dollars, and in addressing neuropathic pain of the cornea which currently lacks a treatment sanctioned by the FDA, these promising outcomes mark a significant advance for the company.
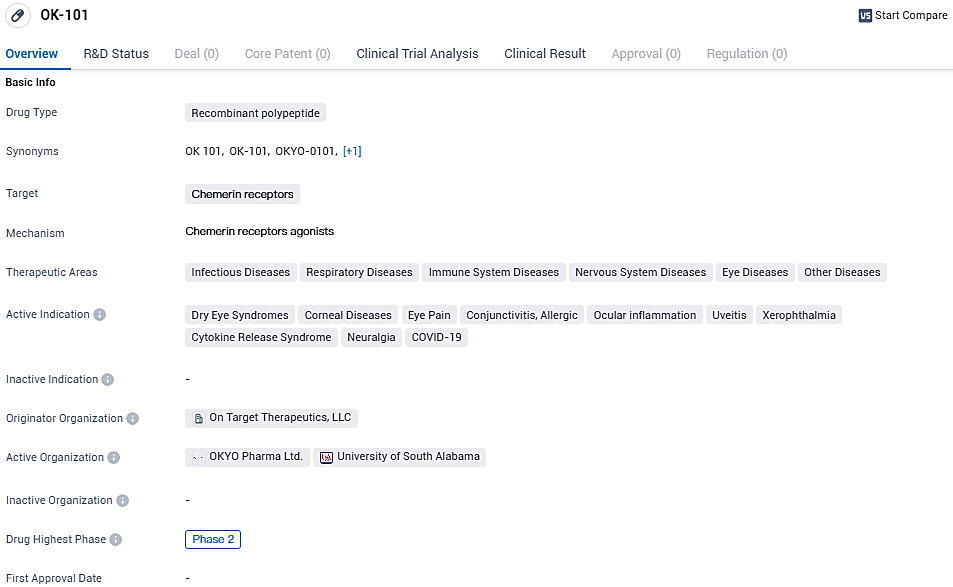
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The initial clinical evaluation of OK-101 in humans forged a definitive, well-informed trajectory for its continued progress toward Phase 3 registration studies. At six American locations, the blinded, randomized, placebo-controlled Phase 2 study was conducted, with participation from 240 individuals suffering from DED, who received the treatment bid.
"In this inaugural human exploration with OK-101 eye drops, an assessment encompassing all participants who were randomized disclosed a durable, statistically noteworthy enhancement across various symptoms associated with dry eye, starting from day 15. Furthermore, there was an evident change in one of the established signs, complete conjunctival lissamine green staining, by the 29th day," commented Jay Pepose, M.D., Ph.D., the founder and medical chief at Pepose Vision Institute and a Clinical Ophthalmology professor at Washington University's School of Medicine in St. Louis.
"I'm filled with immense pride for our team's flawless performance in concluding the OK-101 study before the year's closure, and I extend my gratitude to our committed research sites and the leading experts in the field," stated Gabriele Cerrone, the Non-Executive Chairman of OKYO Pharma. "We've successfully undertaken a Phase 2 evaluation of a groundbreaking agent never before tried in humans, revealing potent signs of efficacy that justify its subsequent examination in an expanded clinical trial."
OKYO's executive team is preparing to organize a briefing via conference call to delve into the study findings once an extensive review of the collected data, including other markers and manifestations of dry eye disease as well as results within subgroups of patients with more severe conditions at the outset, is completed. The briefing call is scheduled to occur in the first quarter of 2024.
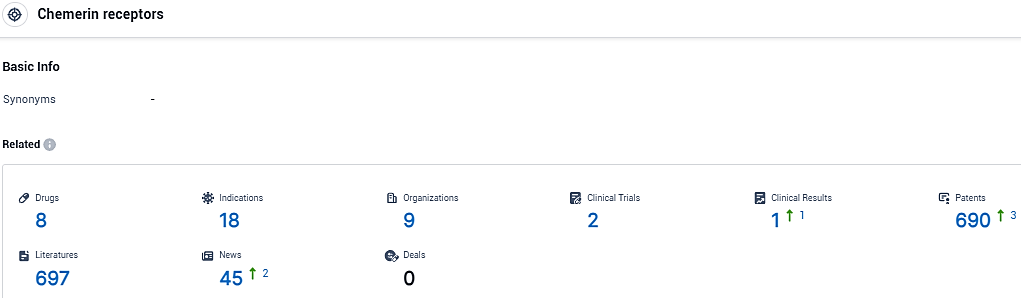
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 12, 2024, there are 8 investigational drugs for the chemerin receptors, including 18 indications, 9 R&D institutions involved, with related clinical trials reaching 2, and as many as 690 patents.
OK-101 is a recombinant polypeptide drug that targets chemerin receptors. It has a wide range of therapeutic areas and is indicated for the treatment of various conditions, including dry eye syndromes, corneal diseases, eye pain, conjunctivitis, ocular inflammation, uveitis, xerophthalmia, cytokine release syndrome, neuralgia, and COVID-19. OK-101 showed clear statistical significance in multiple endpoints in a recently completed Phase 2, multi-center, double-blind, placebo-controlled trial to treat dry eye disease.