Bicycle Therapeutics Unveils Zelenectide Pevedotin Data, Plans NECTIN4-Based Development
Bicycle Therapeutics plc (NASDAQ: BCYC), a pharmaceutical firm leading the development of a novel and distinct category of therapies utilizing its unique bicyclic peptide (Bicycle®) technology, has revealed data on the improved anti-tumor effectiveness of zelenectide pevedotin as a standalone treatment for breast cancer patients exhibiting NECTIN4 gene amplification. This information was shared during the 2024 San Antonio Breast Conference Symposium (SABCS) held in San Antonio, Texas.
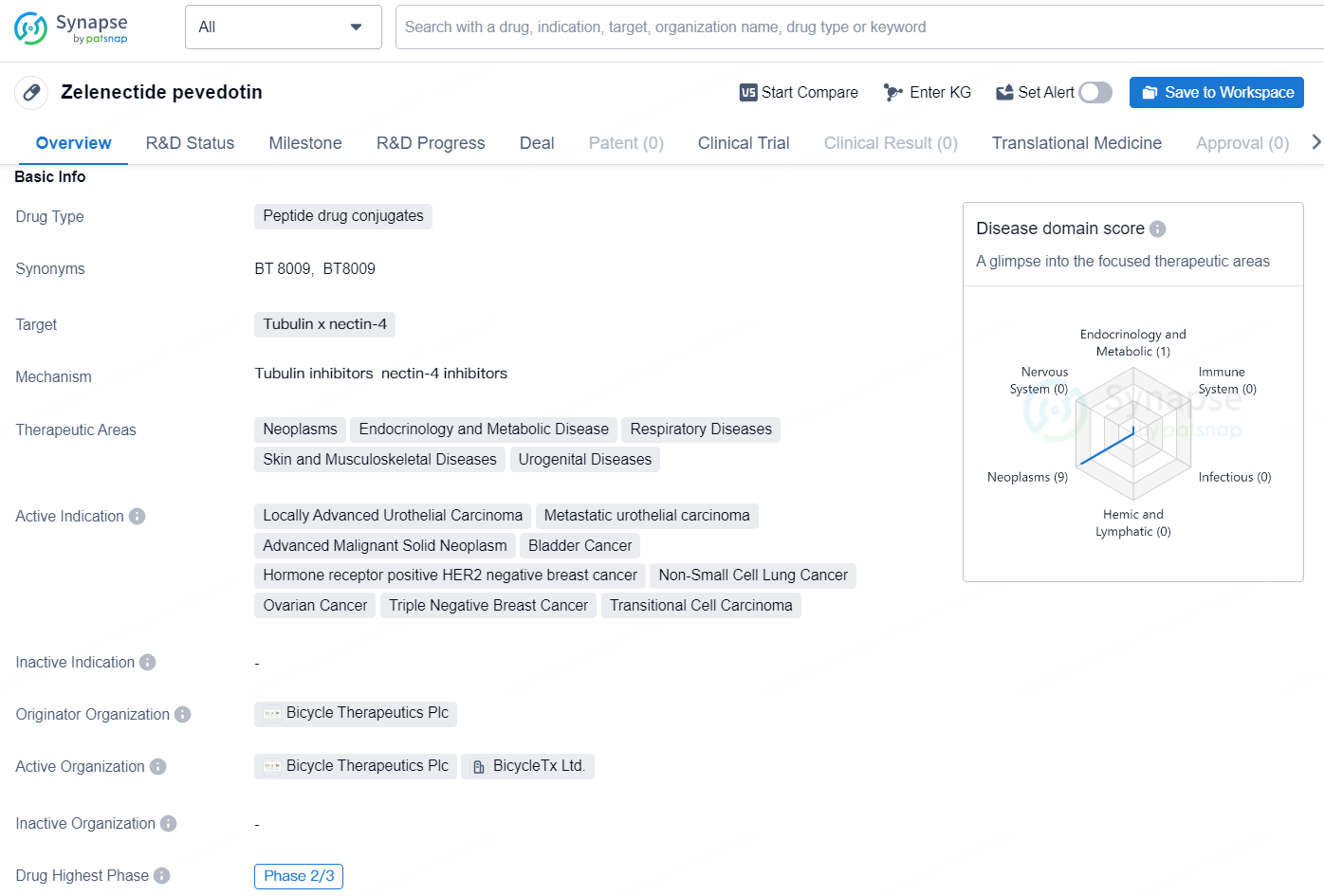
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The company has released preliminary combination results for zelenectide pevedotin paired with pembrolizumab in patients with metastatic urothelial cancer (mUC) who are ineligible for cisplatin and have not received prior treatment (first-line). Additionally, updates were provided regarding enrollment and timelines for the Phase 2/3 Duravelo-2 trial. Furthermore, topline results for zelenectide pevedotin monotherapy in non-small cell lung cancer (NSCLC) patients with NECTIN4 gene amplification were disclosed. Bicycle Therapeutics has scheduled a conference call and webcast for tomorrow, December 13, at 8 a.m. ET to discuss the updates related to zelenectide pevedotin and the development strategy focusing on NECTIN4 gene amplification. The management team will be joined by oncology specialists Dr. Sherene Loi from the Peter MacCallum Cancer Centre in Melbourne, Australia, and Dr. Niklas Klümper from University Hospital Bonn in Germany.
"The comprehensive data shared today enhances the already extensive information on zelenectide pevedotin. We believe that our ambitious development strategy, which utilizes NECTIN4 gene amplification, establishes Bicycle as a frontrunner in targeting Nectin-4 associated cancers," stated Kevin Lee, Ph.D., CEO of Bicycle Therapeutics. "We are optimistic about the topline data for zelenectide pevedotin in combination with pembrolizumab in first-line mUC patients, as it suggests that the response data align with other drug conjugates for treating mUC while maintaining a distinct safety and tolerability profile. Moreover, we are pleased with the progress in our Duravelo-2 registrational trial for zelenectide pevedotin in mUC and anticipate sharing dose selection and topline data in the latter half of next year."
Dr. Lee added, "Though it's still early, the monotherapy results for zelenectide pevedotin in breast cancer and NSCLC patients with NECTIN4 gene amplification highlight its potential anti-tumor activity and establish our next steps in its development. By utilizing NECTIN4 gene amplification, we aim to pinpoint patients who will benefit most from zelenectide pevedotin and speed up development for solid tumor indications beyond bladder cancer. In 2025, we intend to initiate Phase 1/2 trials assessing zelenectide pevedotin in NECTIN4 gene-amplified breast cancer, lung cancer, and several other cancers."
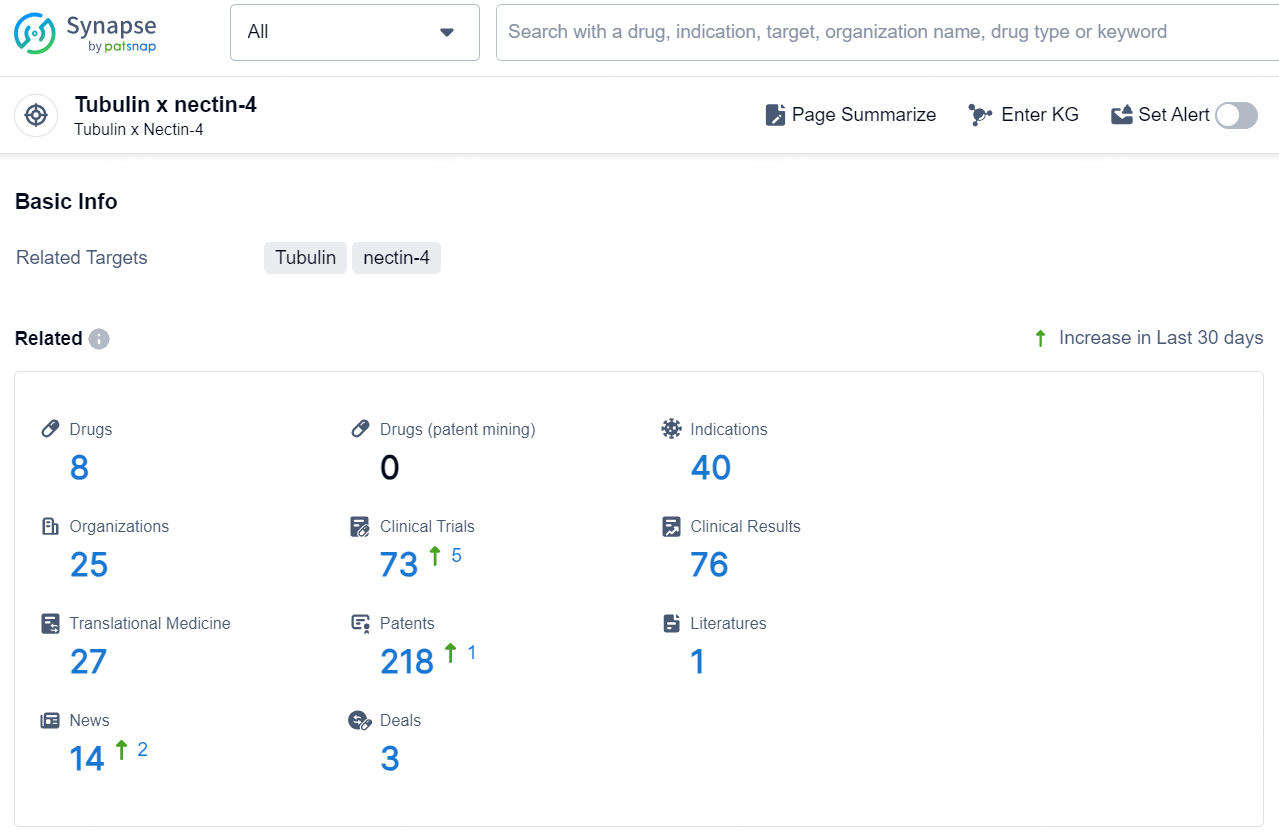
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 18, 2024, there are 8 investigational drugs for the Tubulin x nectin-4 target, including 40 indications, 25 R&D institutions involved, with related clinical trials reaching 73, and as many as 218 patents.
Zelenectide pevedotin is a peptide drug conjugate that targets tubulin and nectin-4. It has shown potential for the treatment of various therapeutic areas including neoplasms, endocrinology and metabolic disease, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases.