First Participant Dosed in RS Oncology's Phase 2 Trial of RSO-021 for Chest Tumor and Lung Metastases
RS Oncology, a biotech firm in the clinical development phase, dedicated to creating groundbreaking treatments to eliminate mesothelioma along with various other medical conditions, has recently reported the initiation of treatment in the premier participant of the Phase 2 dose-escalation segment within its multi-institutional trial based in the UK.
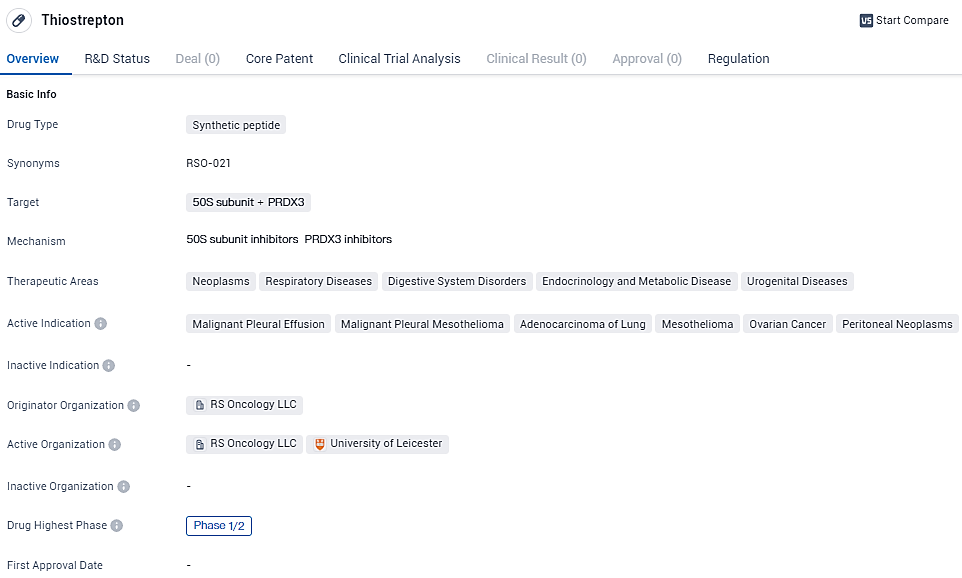
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Accumulation of excess fluid in the pleural cavity, known as malignant pleural effusion (MPE), is a prevalent condition in individuals with various types of cancer and is often associated with a high degree of morbidity among those it affects.
The new anticancer therapy developed by RSO, referred to as RSO-021, is injected into the pleural region on a weekly schedule once the pleural effusion is drained using a permanently implanted catheter. RSO has successfully concluded the initial phase of its MITOPE study, which was designed to increase dosage incrementally, and has determined an optimal dosage for Phase 2, in addition to assessing the safety and tolerability of RSO-021 for patients experiencing a recurrence.
“Treating mesothelioma has consistently posed challenges, largely due to the fact that it's often not identified until it's in an advanced phase,” stated Prof. Dean Fennell, the head of the Mesothelioma Research Programme at Leicester University Hospitals. “We have reasons to be optimistic about RSO-021 due to its distinct method of attacking cancer cells, which holds the promise of new therapeutic options for those fighting this illness.”
Jarrett Duncan, the Chief Executive Officer at RS Oncology, expressed excitement about RSO-021, considering it to be an innovative cancer-fighting agent with the potential to assist cancer patients worldwide who have exhausted their treatment alternatives. "We are marking an important event for both patients and their support systems as we initiate the second phase of our clinical trial," he remarked.
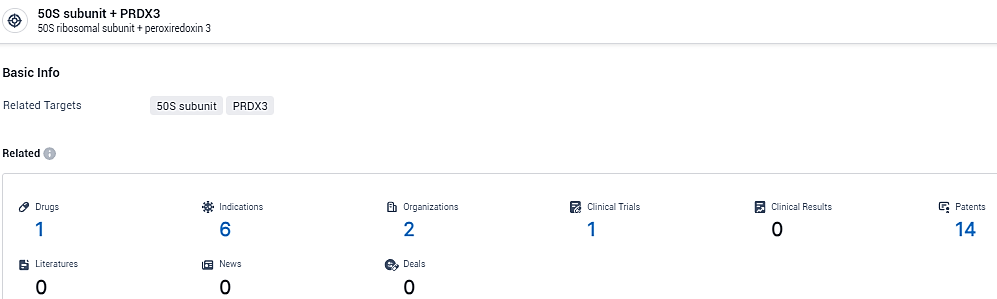
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 7, 2024, there are 1 investigational drugs for the 50S subunit and PRDX3 target, including 6 indications, 2 R&D institutions involved, and as many as 14 patents.
RSO-021 is a novel small molecule treatment that irreversibly binds mitochondrial peroxiredoxin 3 (PRX3). Preclinical studies with RSO-021 have shown that inhibition of the antioxidant signaling network results in selective killing of malignant cells by upregulating oxidative stress; in contrast, healthy cells are spared. The orphan drug designation suggests that Thiostrepton may address unmet medical needs for specific patient populations. However, additional information regarding the drug's mechanism of action, clinical trial results, and potential side effects would be necessary to provide a comprehensive analysis.