Unveiling Escitalopram: How to Search for it on Synapse
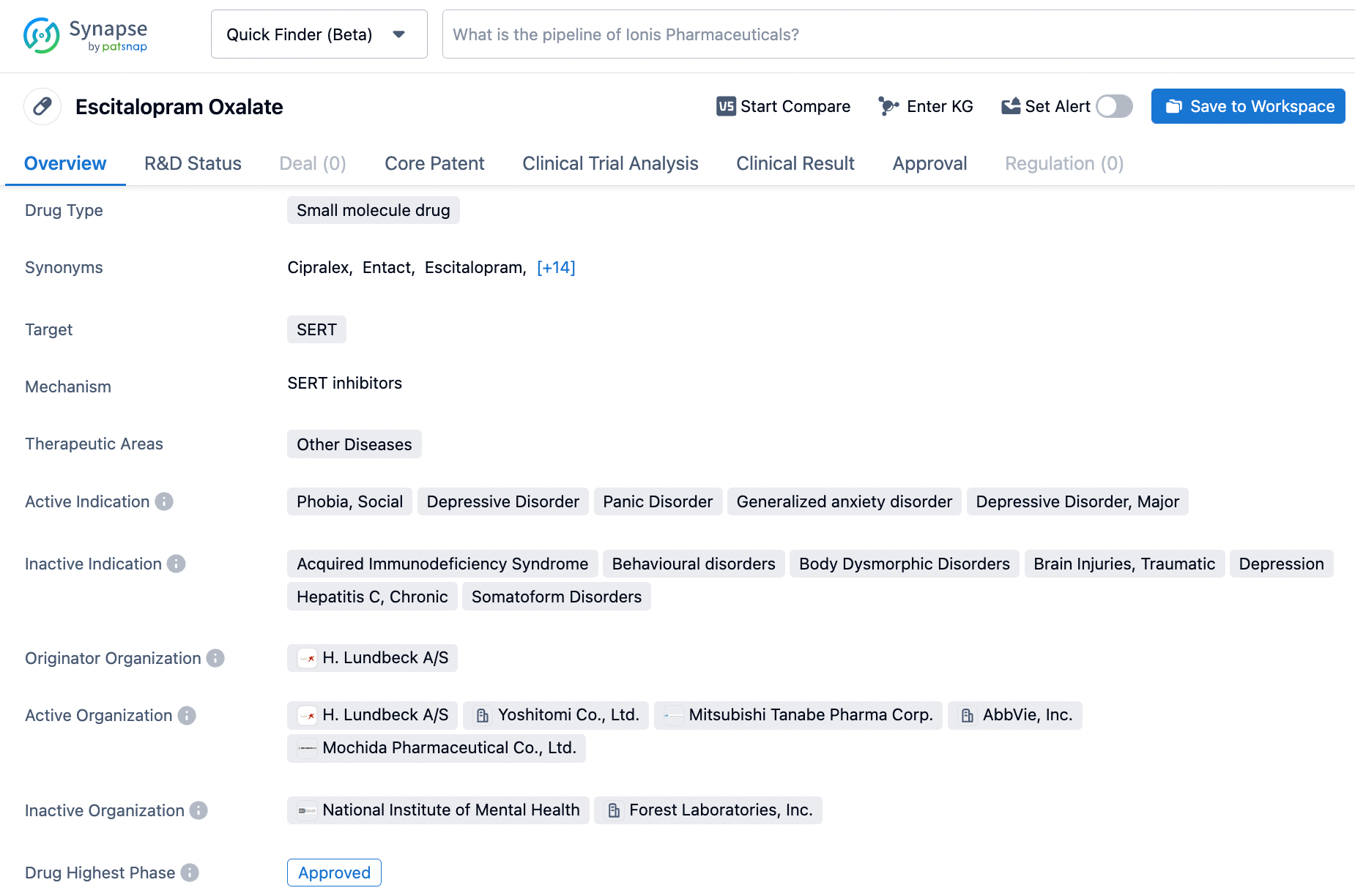
Escitalopram, marketed under the trade name Esertia, is an orally administered selective serotonin reuptake inhibitor. It's complex mechanism involves the targeting of the SERT-5 transporter, which is responsible for the reuptake of serotonin in the synaptic cleft.Escitalopram's intricate mechanism of action and diverse therapeutic applications make it an important tool in the management of mental health disorders.The development of Escitalopram was done by H. Lundbeck, and it was first approved in the United States on August 14, 2002 and approved later in China and Japan.Escitalopram occurs as a fine, white to slightly-yellow powder.Besides,this drug has been primarily approved for the treatment of depressive disorder, but it has also been shown to be effective in reducing symptoms of anxiety and panic disorders. Click on the image below to begin the exploration journey of Escitalopram through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!