Strategy for Retrieving Bispecific Antibody Sequences
Bispecific antibodies (BsAbs) have two distinct binding domains that can bind to two antigens or two epitopes (an antigen part) of the same antigen simultaneously. Using the Patsnap Bio Sequence Database for bispecific antibody sequence retrieval is recommended. This database covers comprehensive literature and publicly disclosed antibody sequences, with a separate module for antibody sequence retrieval. Here are the steps to access bispecific antibody sequences.
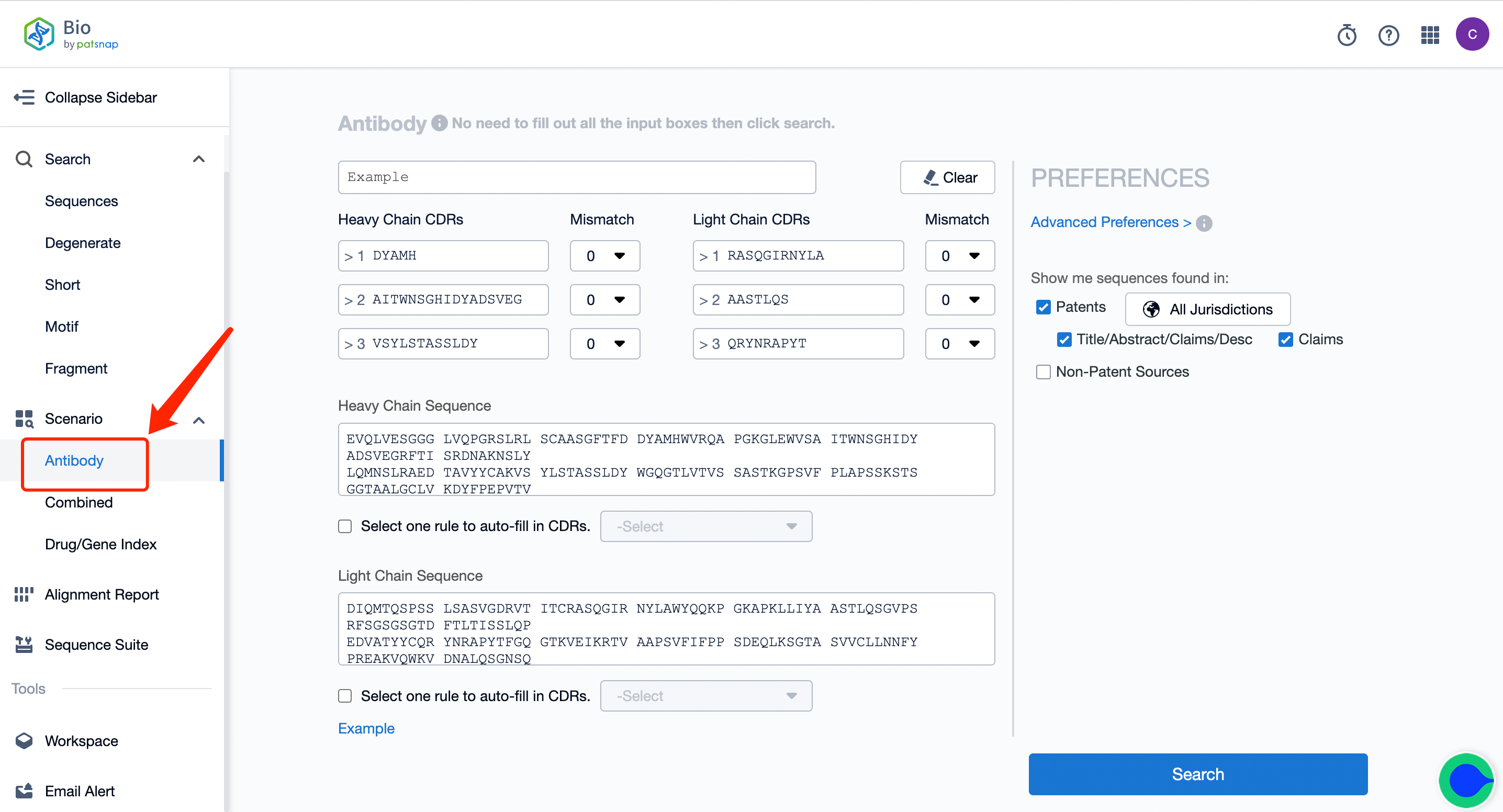
1. First, click on the link to apply for a free account registration in the Bio database. Click "Antibodies" in the left sidebar and enter the antibody sequence retrieval. Input the light/heavy chain/variable region sequence or CDR sequence of one of the target A antibodies of the bispecific antibody.
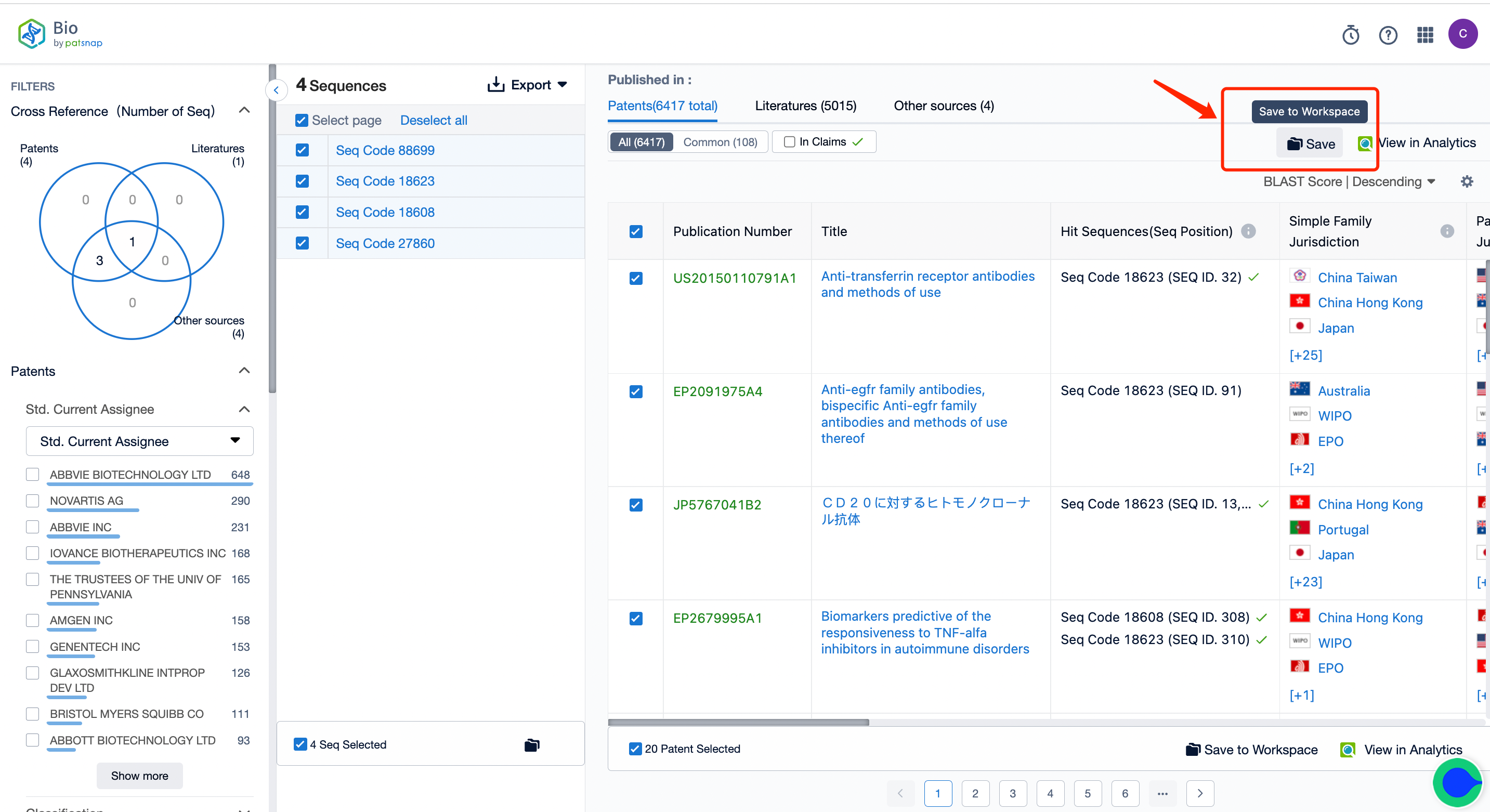
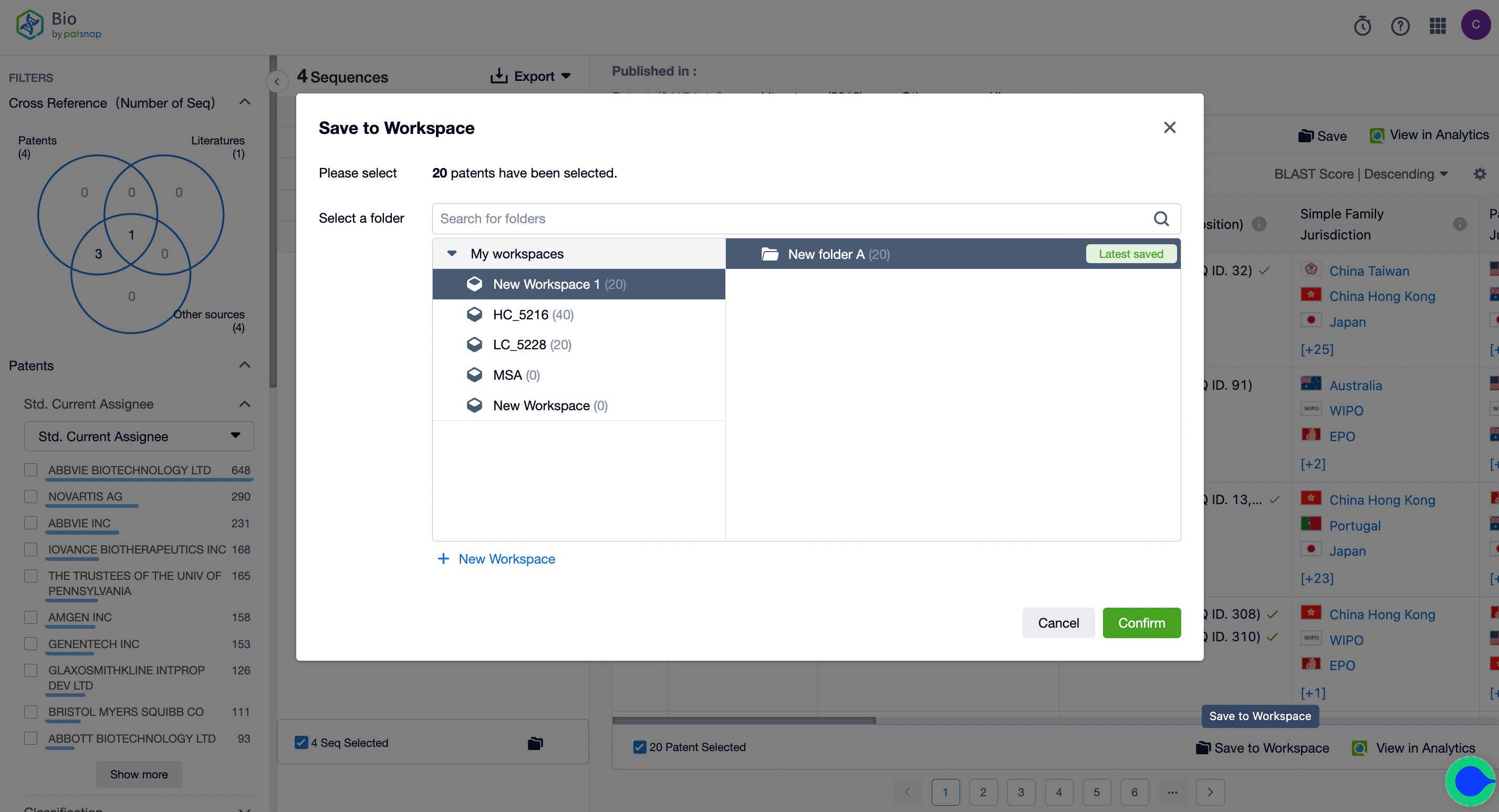
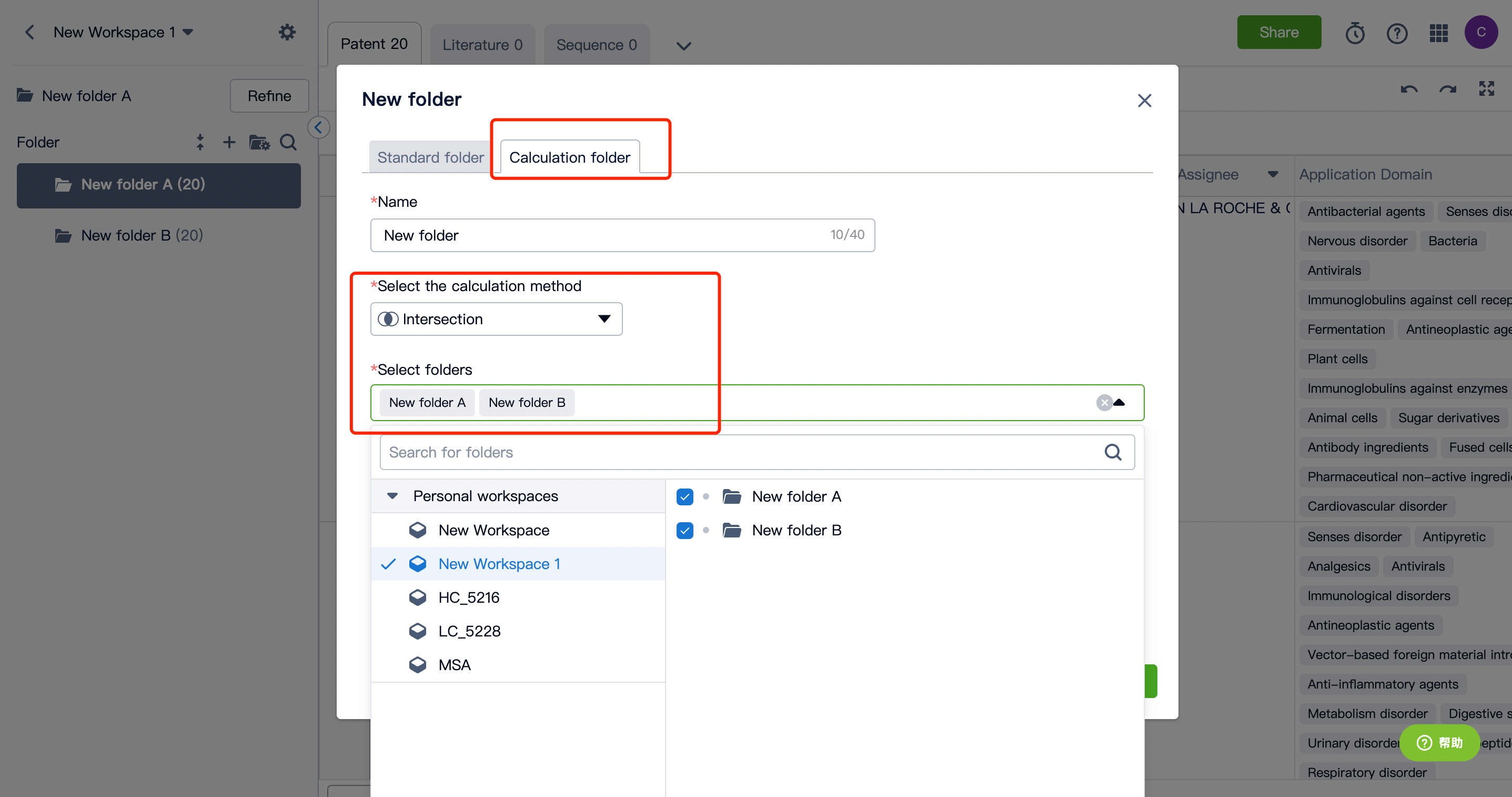
2. Begin the search and keep intersection results in your workspace, creating a new folder labeled "A."
3. In the same manner, retrieve the light/heavy chain/variable region sequence or CDR sequence of target B antibody, and name the search results as a new folder "B."
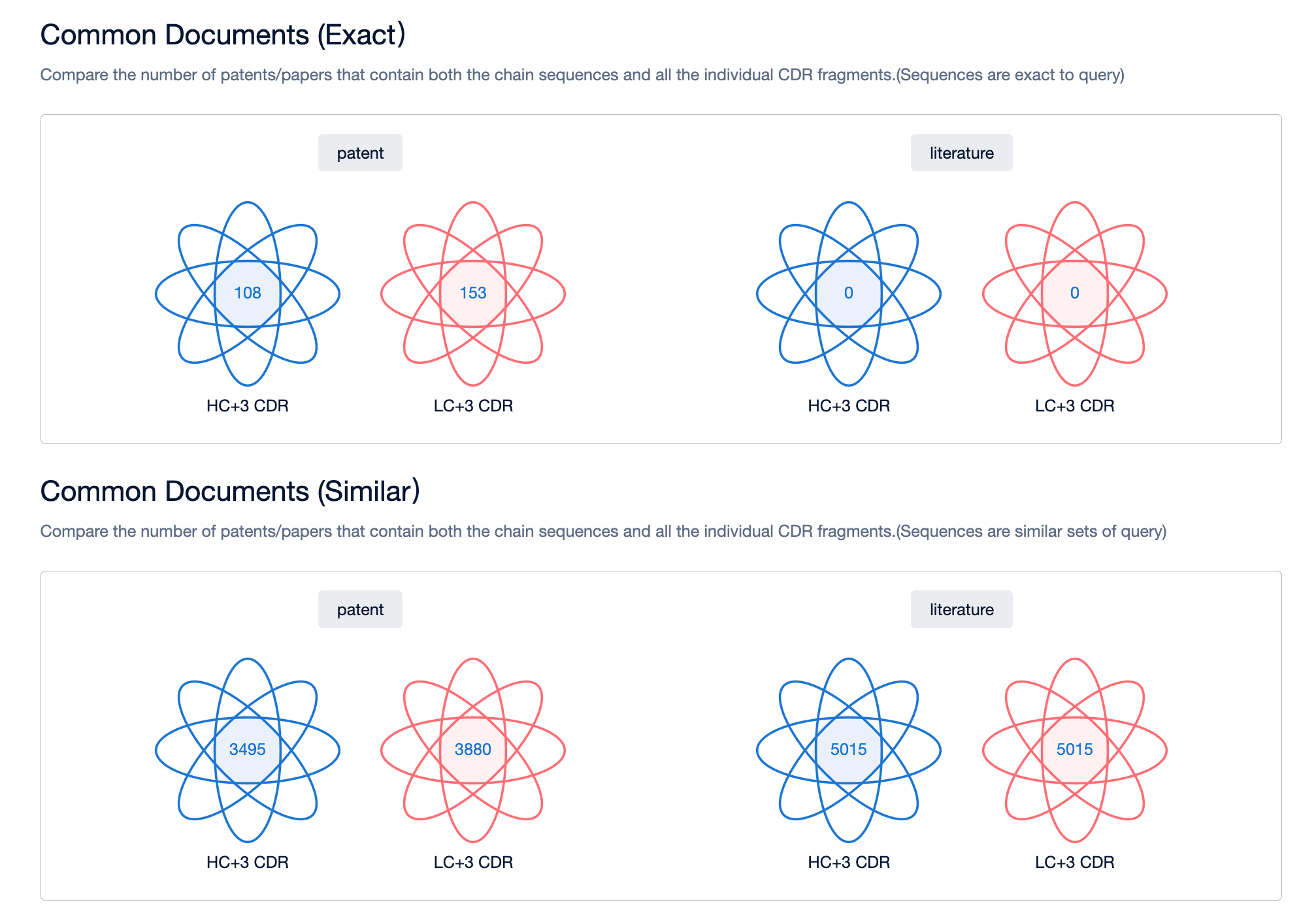
4. By taking the patent intersection of folders A and B, you can obtain patents related to bispecific antibody sequences. If necessary, you can also utilize keywords for further filtering.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.