"Super drugs" for treating cancer and AIDS: Chemokines family
Chemokines, also known as chemotactic cytokines, are a group of factors that, in conjunction with their cell surface receptors, induce the directed migration of leukocytes. They are produced in response to inflammatory cytokines, growth factors, and pathogenic stimuli. Chemokine signaling leads to the transcription of target genes, involving cell invasion, movement, interaction with the extracellular matrix (ECM), and survival. Chemokine signals can coordinate cell movement during inflammation and steady-state transport of hematopoietic stem cells, lymphocytes, and dendritic cells. They may also control the movement of other types of cells, such as adult stem cells and endothelial cells. In cells expressing appropriate numbers of chemokine receptors, directed migration occurs along the chemical gradient of ligands (known as the chemokine gradient), causing cells to move toward areas with high local concentrations of chemokines.
The Role of Chemokines and Their Receptors in Cancer
Chemokines play a crucial role in inflammation and immunity and are key mediators in cancer-associated inflammation. They have the ability to directly affect the proliferation and metastasis of cancer cells, with specific functions as follows:
Leukocyte Recruitment - The correct movement of immune cells is coordinated by the temporal and spatial expression of chemokines. Inflammatory CC (CCL2, CCL3, CCL5) and CXC (CXCL1, CXCL2, CXCL5, CXCL6, and CXCL8) chemokines recruit CCR2+ monocytes and CXCR2+ neutrophils at tumor sites, differentiating into tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs), exerting either pro-tumor or anti-tumor effects. Certain chemokines present at the tumor site can alter the activation of leukocytes, for instance, CXCL16 acting on CXCR6 induces polarization of macrophages towards a pro-tumor phenotype in solid tumors. CXCL9 and CXCL10 are closely associated with Th1 immune responses by recruiting NK cells, CD4+ Th1 and CD8+ cytotoxic lymphocytes, potentially inducing anti-tumor responses. Additionally, CCL20, CCL5, and CXCL12 are effective attractants for dendritic cells; CCL21 and CCL19 not only recruit CCR7+ DCs, but also regulatory T cells (Tregs). CCL17 and CCL22, acting on CCR4, can directly recruit Tregs and Th2 lymphocytes, promoting tumor growth and proliferation.
Angiogenesis - CC and CXC chemokines play crucial roles in tumor angiogenesis, which is vitally important for tumor growth and metastasis. Depending on the presence of N-terminal glutamic acid-leucine-arginine (ELR) motif, CXC chemokines are divided into ELR+ chemokines that promote angiogenesis and ELR- chemokines that inhibit angiogenesis. CCL2, CCL11, CCL16, CCL18, and CXCL8 promote tumor angiogenesis and endothelial cell survival. Moreover, the interaction between CXCL16 and CXCR6 is a potent angiogenic mediator. CXCL12 and CCL2 promote angiogenesis and inhibit endothelial cell apoptosis either by directly binding to CXCR4 and CCR2 receptors expressed on tumor vasculature, or indirectly promoting leukocyte recruitment. In contrast, chemokines such as CCL21 and ELR- chemokines (CXCL4, CXCL9, CXCL10, and CXCL11) inhibit angiogenesis and endothelial cell proliferation.
Tumor Growth and Proliferation - The chemokines produced by the tumor cells themselves can directly promote cancer cell proliferation by binding to chemokine receptors transiently expressed on tumor cells, activate different signaling pathways, such as PI3K/AKT/NF-kB and MAPK/ERK pathways. Furthermore, they can promote tumor cell survival by inhibiting tumor cell apoptosis and regulating the balance between pro-apoptotic and anti-apoptotic molecules (e.g., down-regulating the expression of Bcl-2 or inhibiting the activation of caspase-3 and caspase-9).
Metastasis - The chemokine receptors expressed by cancer cells promote their migration to metastatic sites. Several chemokines and chemokine receptors are involved in this phenomenon: CCR7 mediates tumor cell migration to lymph nodes, where its ligands CCL19 and CCL21 are produced; The CCR10/CCL27 axis promotes adhesion and survival of melanoma cells during metastatic spread; CCL28 promotes breast cancer growth and metastasis via the MAPK/ERK pathway; Chemokine receptor CXCR5 and its ligand CXCL13 promote bone metastasis of prostate cancer. In some tumors, the expression of CXCR4 endows cancer cells with the ability to migrate and metastasize to organs that secrete high levels of CXCL12.
Immunotherapeutic Strategies Utilizing Chemokines and Their Receptors
Chemokines and their receptors, as mediators of immune cell transportation, are involved in all stages of tumor development and play a crucial role in the composition of the tumor microenvironment, demonstrating both pro-tumoral and anti-tumoral effects. Generally, there are two methods of using chemokines and their receptors in immunotherapy:
① Targeting Protumoral Chemokines: Various strategies utilizing or targeting chemokines have been established, and new drug candidates are undergoing clinical trials for validation. It should be noted that chemokines and their receptors are involved in the biological aspects of cancer and targeted drug therapies.
② Increasing Concentrations of Antitumoral Chemokines: Either as standalone treatments or in combination with other therapeutic strategies to enhance the recruitment and activation of endogenous effector cells, leading to a mediated effective anti-tumor immune response. Currently, strategies that combine anti-tumor chemokines with cancer vaccines, oncolytic viruses (OVs), and CAR-T cell therapies have recently been established and are now being explored in phase I and phase II clinical trials.
In summary, the strategy of using anti-tumor therapeutic drugs, while simultaneously maximizing the use of chemokines and interleukins to mobilize endogenous immune cells, is a promising approach for the future.
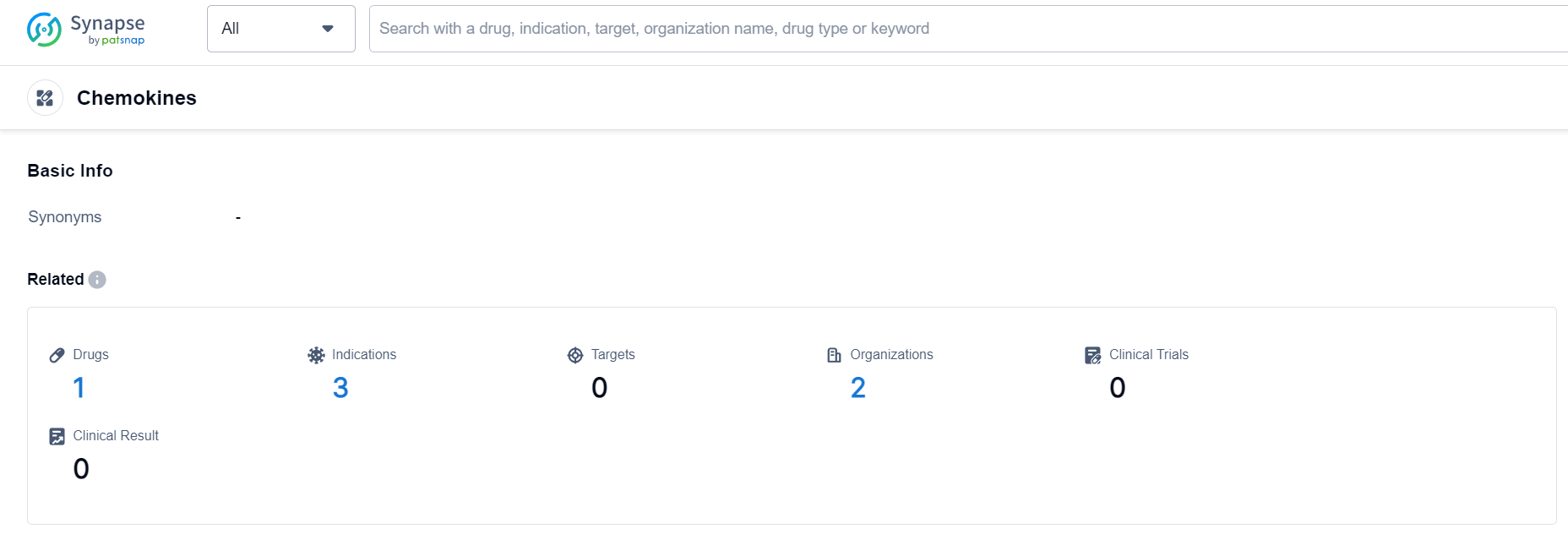
Chemokines Competitive Landscape
According to Patsnap Synapse, as of 19 Sep 2023, there are a total of 1 Chemokines drugs worldwide, from 2 organizations, covering 0 targets, 3 indications, and conducting 0 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this drug type.
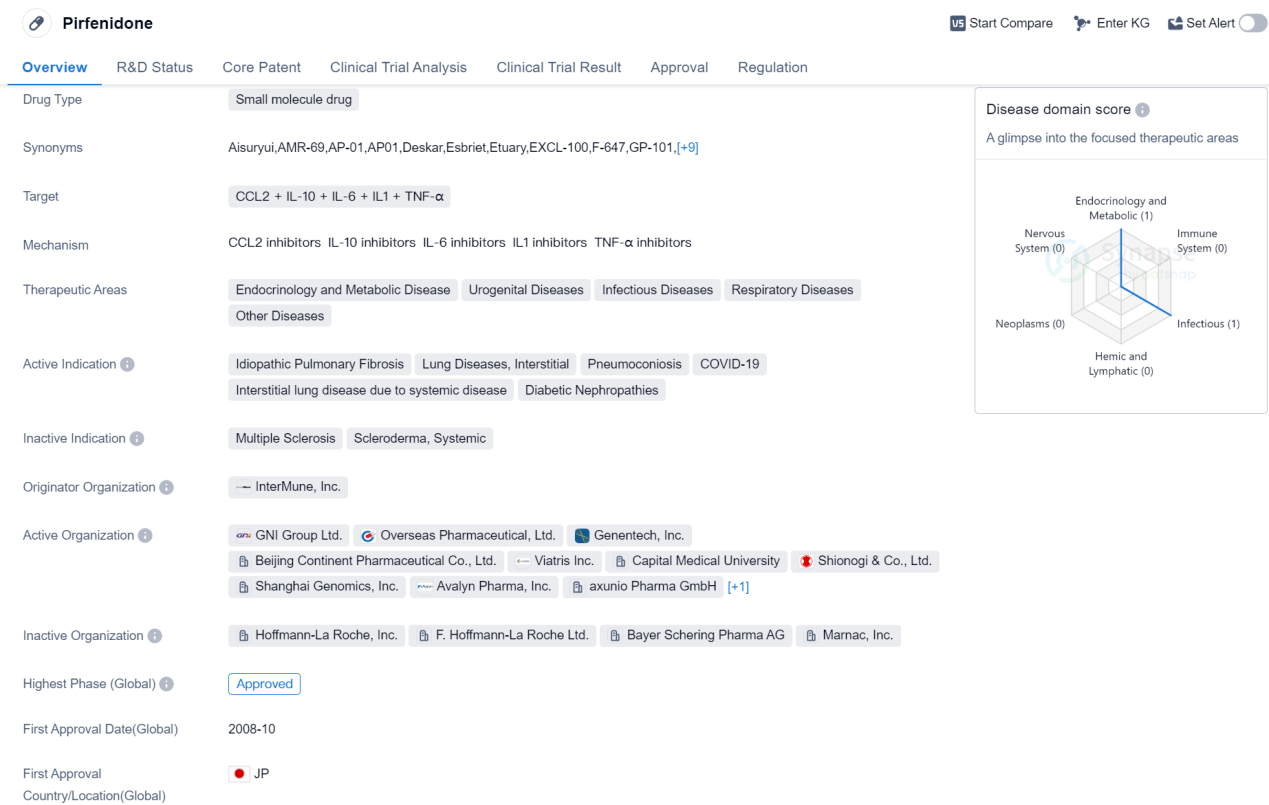
Approved of Chemokines Medicinal: Pirfenidone
Pirfenidone is a small molecule drug that has been developed to target multiple factors involved in various diseases. It acts on CCL2, IL-10, IL-6, IL1, and TNF-α, which are known to play a role in the pathogenesis of several conditions. The drug has shown potential therapeutic benefits in a range of therapeutic areas, including endocrinology and metabolic disease, urogenital diseases, infectious diseases, respiratory diseases, and other diseases.
Pirfenidone has been approved for the treatment of idiopathic pulmonary fibrosis, a progressive lung disease characterized by scarring of the lung tissue. It has also been indicated for lung diseases with interstitial involvement, pneumoconiosis, COVID-19, interstitial lung disease due to systemic disease, and diabetic nephropathies.
The drug was originally developed by InterMune, Inc., and it received its first approval in Japan in October 2008. Since then, it has gained approval in other countries as well. Pirfenidone has been granted special regulatory designations, including orphan drug status, fast track designation, and breakthrough therapy designation. These designations are given to drugs that show promise in treating rare diseases, have the potential to address unmet medical needs, or demonstrate significant advantages over existing treatments.
The approval of Pirfenidone in multiple countries and its regulatory designations highlight its potential as a valuable treatment option for various diseases. Its mechanism of action, targeting multiple factors involved in disease progression, makes it a promising drug in the field of biomedicine. The drug's approval for idiopathic pulmonary fibrosis, a condition with limited treatment options, further emphasizes its importance in addressing unmet medical needs.
In summary, Pirfenidone is a small molecule drug developed by InterMune, Inc. It targets multiple factors involved in disease progression and has been approved for the treatment of idiopathic pulmonary fibrosis and other related conditions. Its regulatory designations and global approvals signify its potential as a valuable therapeutic option in the pharmaceutical industry.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
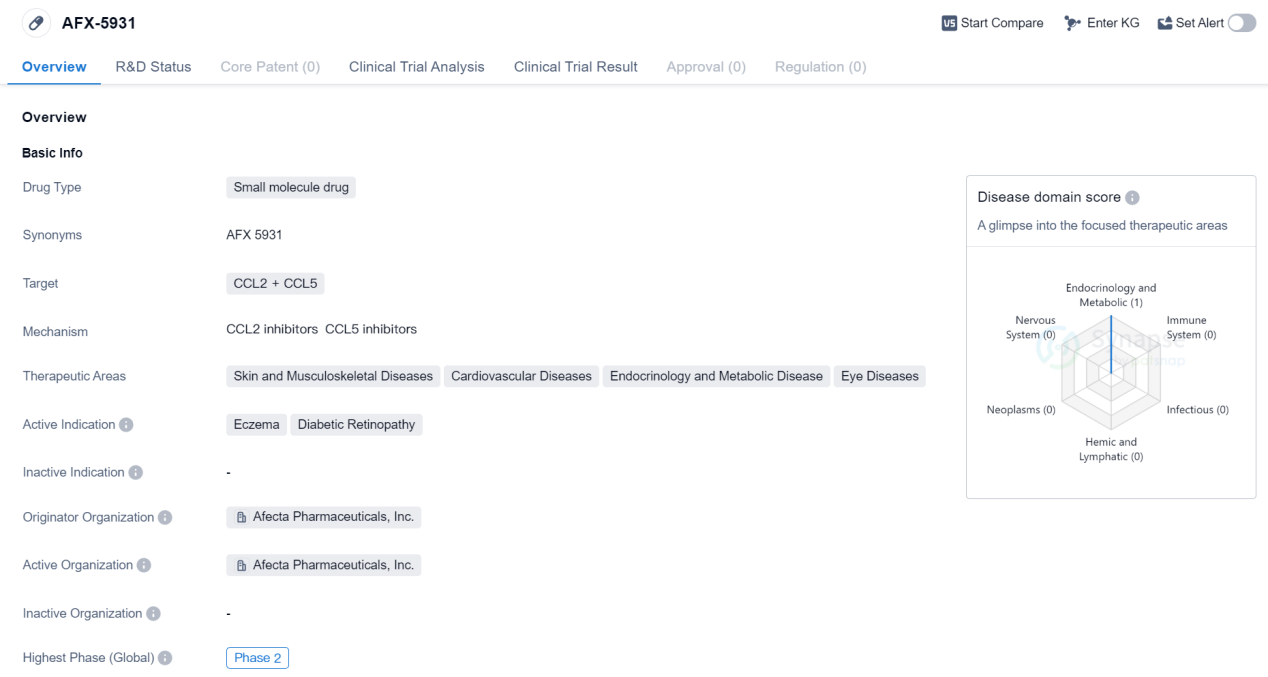
Phase II Clinical Trial of Chemokines Medicinal: AFX-5931
AFX-5931 is a small molecule drug developed by Afecta Pharmaceuticals, Inc. It targets the proteins CCL2 and CCL5 and is currently in Phase 2 of clinical trials. The drug has shown potential in treating various therapeutic areas including skin and musculoskeletal diseases, cardiovascular diseases, endocrinology and metabolic diseases, and eye diseases. The active indications for AFX-5931 are eczema and diabetic retinopathy.
Eczema is a chronic skin condition characterized by inflammation, itching, and redness. It affects millions of people worldwide and can significantly impact their quality of life. Current treatment options for eczema are limited, and there is a need for more effective and targeted therapies. AFX-5931, with its ability to target CCL2 and CCL5, may offer a promising solution for managing eczema symptoms and reducing inflammation.
Diabetic retinopathy is a complication of diabetes that affects the blood vessels in the retina. It is a leading cause of blindness in adults and can have a significant impact on the quality of life of individuals with diabetes. AFX-5931's potential in treating diabetic retinopathy suggests that it may help prevent or slow down the progression of this condition, ultimately preserving vision in diabetic patients.
The fact that AFX-5931 is currently in Phase 2 of clinical trials indicates that it has already undergone initial testing for safety and efficacy. Phase 2 trials involve a larger number of participants and aim to further evaluate the drug's effectiveness and side effects. This stage of development is crucial in determining whether AFX-5931 can progress to the next phase of clinical trials and eventually be approved for commercial use.
As a small molecule drug, AFX-5931 offers several advantages. Small molecules are typically easier to manufacture, have better stability, and can be administered orally, making them more convenient for patients. These characteristics contribute to the potential commercial viability of AFX-5931 if it proves to be safe and effective in further clinical trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, AFX-5931 is a small molecule drug developed by Afecta Pharmaceuticals, Inc. It targets CCL2 and CCL5 and is currently in Phase 2 of clinical trials. The drug shows promise in treating eczema and diabetic retinopathy, two conditions with significant unmet medical needs. Further clinical trials will determine its safety and efficacy, and if successful, AFX-5931 may offer a new treatment option for patients suffering from these conditions.