Myeloid Therapeutics begins administering MT-302, a new TROP2-focusing RNA CAR, for advanced or metastatic epithelial tumors
Myeloid Therapeutics, Inc., a business involved in clinical-stage cancer treatment, has administered the initial dose of MT-302 to a patient in a Phase 1 trial involving advanced or metastatic epithelial tumors. The firm's primary development candidate for in vivo immune cell programming, MT-302, utilizes TROP2-targeting RNA chimeric antigen receptors that are selectively expressed within myeloid cells.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Administering MT-302 symbolizes a significant progression in creating new treatment options for solid tumors, specifically originating from epithelial cells. In contrast to conventional CAR-T cell therapies, Myeloid offers a novel strategy with a focus on in vivo programming of immune cells utilizing a ready-to-use mRNA encoded CAR technology.
TROP2 is a crucial clinal target, backed by clinical outcomes from several TROP2-centered antibody-drug conjugates. MT-302 enhances previous methodologies by targeting TROP2 and initiating a broad immune response against the tumor. This immune response is critical for long-lasting immune surveillance and defense against tumor reappearance, aligning with Myeloid's overall clinical objective to enhance patient outcomes.
" Initiation of patient dosing with MT-302 marks a significant achievement for Myeloid in our pursuit to offer improved treatment solutions for solid tumor patients," stated Myeloid's CEO, Daniel Getts, Ph.D. "By progressing MT-302 into the clinical stage, we are leveraging the innate immune system's power to overcome observed solid tumor CAR-T limitations. With our Phase 1 study, we look forward to progressing MT-302 and showcasing the potential of our innate immunity platform to program cells directly in vivo and yield better results.”
In the United States, more than 500,000 patients per year are diagnosed with TROP2 expressing tumors. While there has been significant clinical progression in the class of TROP2-centered ADCs, MT-302 takes it one step further by directly targeting the tumor, incorporating immune-stimulation and inducing an adaptive immune reaction to the tumor's neoantigens. This adaptive response is fundamental for long-lasting immune surveillance and defense against the disease.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
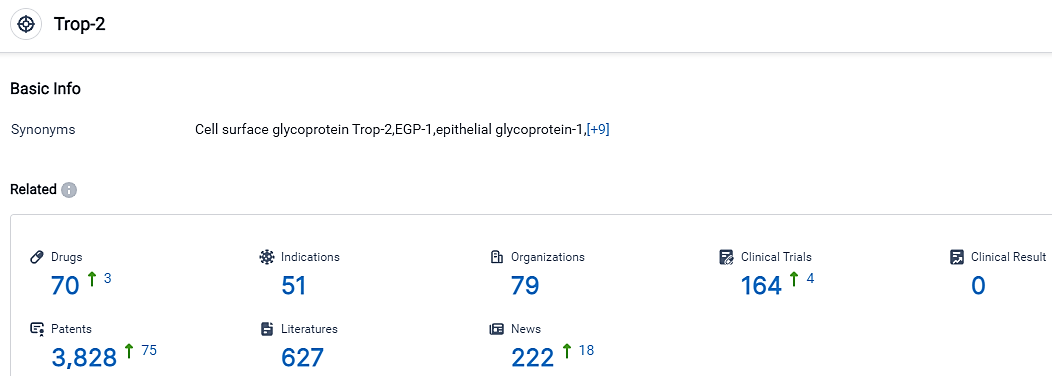
According to the data provided by the Synapse Database, As of September 18, 2023, there are 70 investigational drugs for the Trop-2 target, including 51 indications, 79 R&D institutions involved, with related clinical trials reaching 164,and as many as 3828 patents.
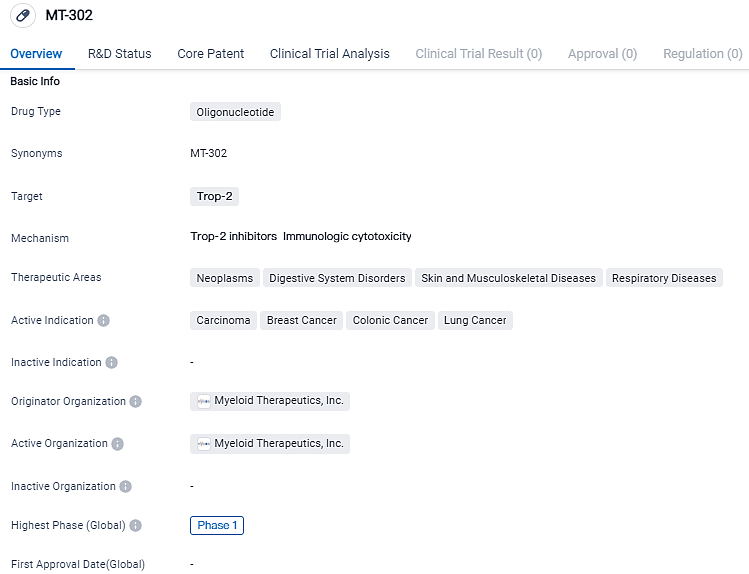
MT-302 represents the first candidate in an entirely new, therapeutic modality. It is a first-in-class, TROP2-FcA-LNP, with a strong preclinical profile that supports its advance into this first-in-human trial. It targets Trop-2 and has shown potential in treating various neoplasms, digestive system disorders, skin and musculoskeletal diseases, and respiratory diseases. With its active indications in carcinoma, breast cancer, colonic cancer, and lung cancer, MT-302 holds promise as a potential therapeutic option for patients in need.