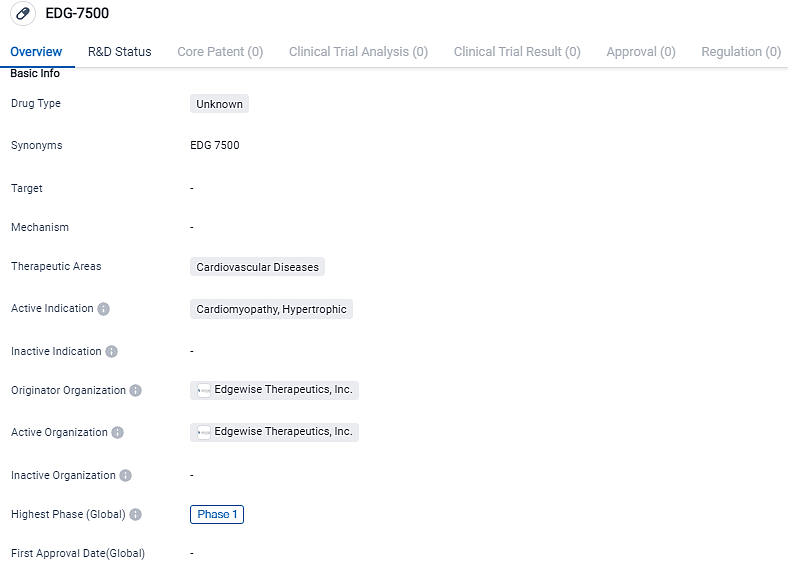
Edgewise Therapeutics commences Phase 1 human trial for EDG-7500, a promising treatment for Hypertrophic Cardiomyopathy and severe Cardiac Diastolic Dysfunction
Edgewise Therapeutics, Inc., a prominent enterprise in the biopharmaceutical industry dealing with muscle disorders, has reported the inaugural administration of EDG-7500 in an initial Phase 1 clinical examination. EDG-7500 is a first-in-class oral treatment, described as a selective modulator of cardiac sarcomeres, specifically engineered to reduce premature contraction speed and treat obstructed cardiac relaxation associated with HCM and other related diastolic dysfunction conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
First phase trial is aimed at evaluating the safety and tolerability parameters of EDG-7500, as well as its pharmacokinetics and pharmacodynamics in healthy adult subjects. Another trial we anticipate to kick-start in the first half of 2024 is a Phase 1b study of EDG-7500 targeted at obstructive HCM afflicted individuals.
Launching our Phase 1 trial for EDG-7500, our inaugural cardiovascular therapeutic endeavour, signifies a crucial point in our company’s progress," stated Marc Semigran, M.D., the Chief Development Officer of Edgewise Therapeutics. "With over three decades of dedication to improving the lives of serious heart disease patients, I am thrilled about the promise of EDG-7500 for those impacted by HCM, an area with considerable unmet requirements."
"We're pleased to include EDG-7500 in our expanding clinical experimentation portfolio," said Kevin Koch, Ph.D., President and Chief Executive Officer of Edgewise Therapeutics, demonstrating our commitment to creating accurate treatments aimed at patients with acute genetic muscle disorders. "As we mark this accomplishment, we're excited to start similar experiments in HCM next year and extensively in diastolic dysfunction conditions."
The Phase 1 trial is designed to be a randomized, double-blind, placebo-controlled study to evaluate the safety and tolerance along with pharmacokinetics and pharmacodynamics of EDG-7500 in healthy adults with a single or multiple ascending doses. Those who participate in this study will be administered a one-time or multiple oral dosage of EDG-7500 or a placebo.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
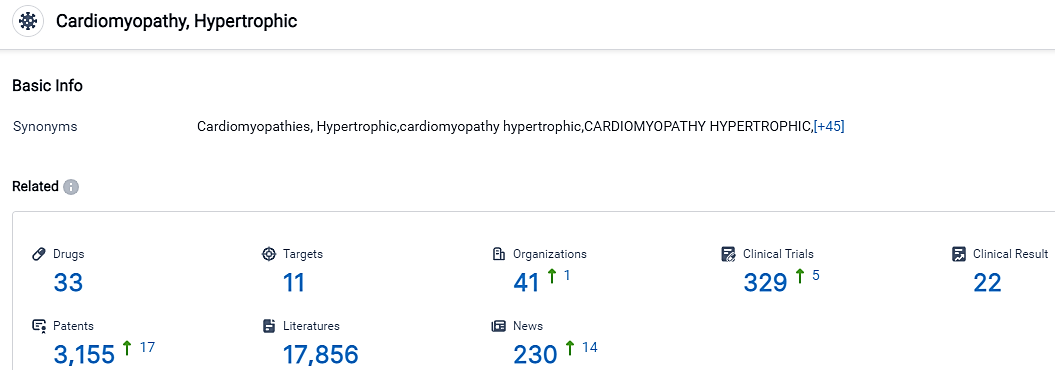
According to the data provided by the Synapse Database, As of September 18, 2023, there are 33 investigational drugs for the Cardiomyopathy Hypertrophic, including 11 targets, 41 R&D institutions involved, with related clinical trials reaching 329,and as many as 3155 patents.
EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator that is advancing in a Phase 1 trial. The compound is designed to slow early contraction velocity and improve impaired cardiac relaxation. The development of this drug highlights the ongoing efforts in the pharmaceutical industry to address unmet medical needs in the field of biomedicine.