BMS Lays Out Strategic Direction in Nuclear Medicine
In early 2024, BMS announced that it had successfully completed the acquisition of RayzeBio. According to the earlier announced proposal by BMS to acquire all the outstanding common shares of RayzeBio, the cash price per share was 62.50 USD, amounting to approximately 4.1 billion USD in total.
With the completion of this acquisition, RayzeBio has brought BMS a promising pipeline of radiotherapeutic drugs, including its key project RYZ101 (225Ac-DOTATATE). It is reported that this drug targets the Somatostatin Receptor 2 (SSTR2) that is overexpressed in gastroenteropancreatic neuroendocrine tumors (GEP-NETs) and extensive stage small cell lung cancer (ES-SCLC). RYZ101 is currently undergoing a Phase III clinical trial, evaluating the efficacy of RYZ101 in patients with SSTR-positive GEP-NETs who were previously treated with Lu-177 somatostatin therapy.
In addition to this, RayzeBio also has two pipelines targeting GPC3 and CA9 respectively.

According to the updated information on BMS's official website, after acquiring RayzeBio, the company is committed to developing the next generation of nuclear medicine. The team believes that alpha emitters are the next generation of therapeutic radiopharmaceuticals and may become the preferred radiopharmaceutical for treating solid tumors due to their favorable chemical properties and decay characteristics, which are superior to beta emitters. Alpha radiation, due to its higher linear energy transfer rate, more frequently causes direct, irreparable double-strand DNA breaks than beta radiation, while its shorter path length allows for the protection of surrounding healthy tissues.
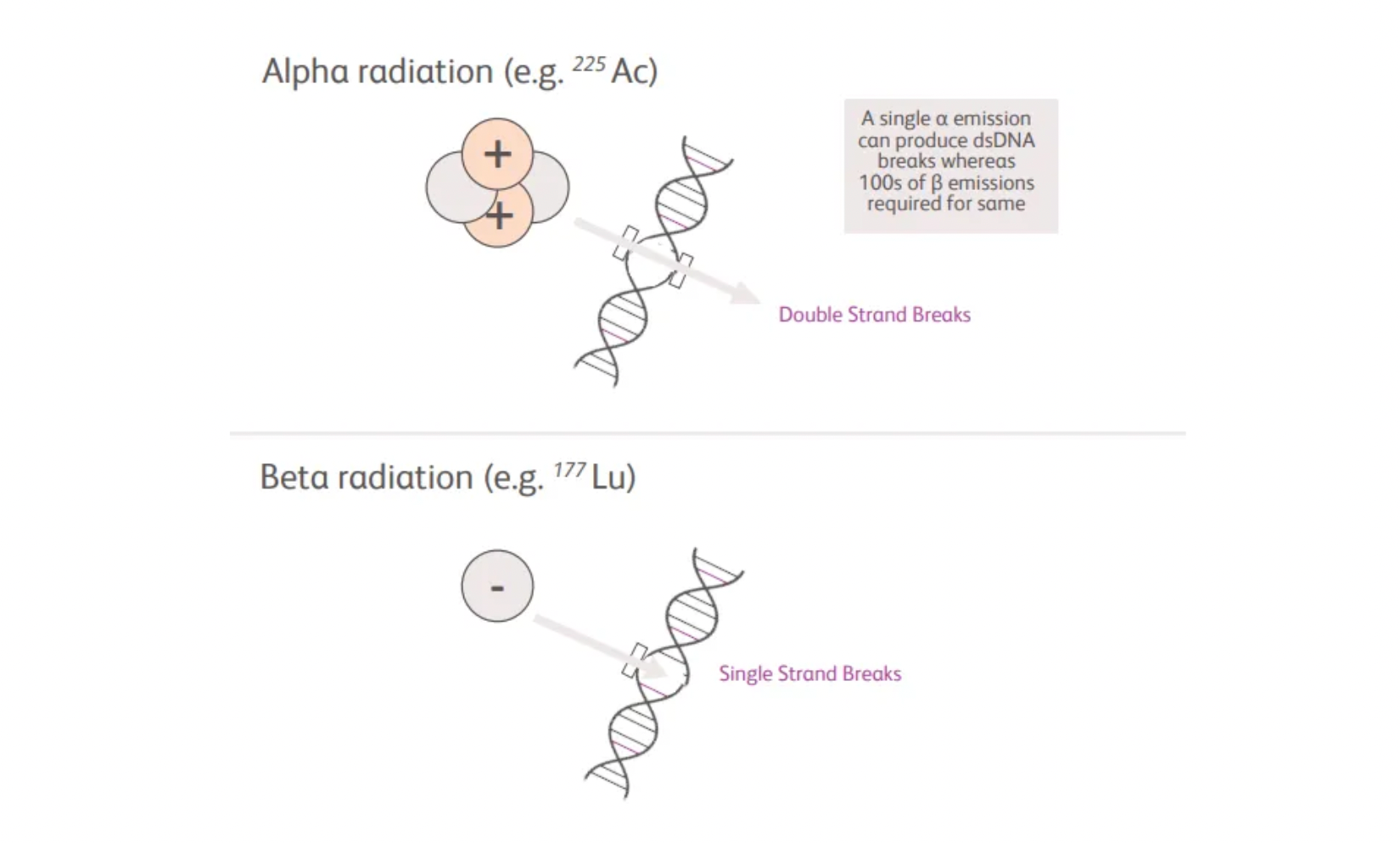
The flagship project of BMS, RYZ101, utilizes Actinium-225 (Ac225), a preferred alpha radiation emitter. Ac225 may enhance the effect of Radiopharmaceutical Therapy (RPT), allowing tumors with low target expression to achieve therapeutic effects, and overcome resistance to beta radiation, thereby potentially bringing better treatment outcomes for patients.
However, the reality of the entire industry is that the field of nuclear medicine is often affected by supply limitations. In June, news came out that due to a shortage of Actinium isotopes, its subsidiary RayzeBio had temporarily halted the enrollment of new patients in its ACTION-1 Phase 3 clinical trials.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!