Boehringer Ingelheim's Adalimumab-adbm is available at low wholesale acquisition cost
Boehringer Ingelheim revealed that their interchangeable* biosimilar to Humira®, known as Adalimumab-adbm injection, is now available at a low wholesale acquisition cost. The medication, approved for treating various chronic inflammatory disorders, carries an 81% price cut compared to Humira. Additionally, it is marketed under the label name Cyltezo®, which was introduced in July 2023 and comes with a 5% price discount on Humira.
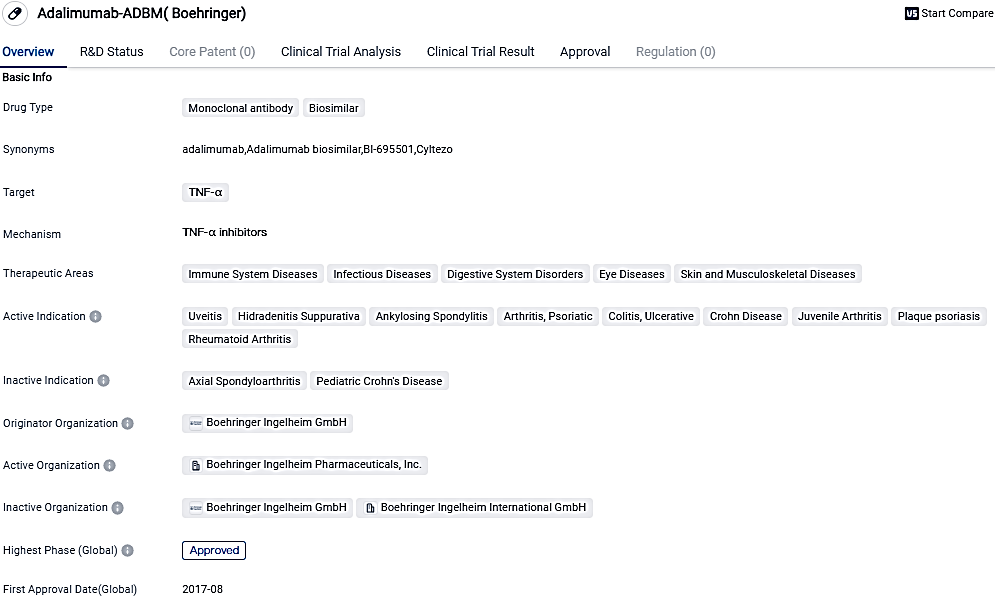
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Obtaining reasonably priced medications remains a continuous struggle for numerous people, which is the driving force behind our commitment to making our products easily obtainable," expressed Stephen Pagnotta, Executive Director and Biosimilar Commercial Lead at Boehringer Ingelheim.
"Biosimilars are designed to play a significant role in guaranteeing the financial sustainability of healthcare systems. We hope that our dual pricing strategy can bolster that sustainability, potentially enhancing accessibility of Adalimumab-adbm and cater to diverse requirements of those living with various chronic inflammatory ailments." Stephen Pagnotta added.
Adalimumab-adbm, which can be used as a replacement for Humira, is a citrate-free formulation. It is available in 40 mg/0.8 mL, 20 mg/0.4 mL and 10 mg/0.2 mL pre-filled syringes, and also as a 40 mg/0.8 mL pre-filled autoinjector.
Boehringer Ingelheim is among the global leaders in biologic medicine production, manufacturing biologic drugs to support our wide-ranging pipeline, as well as catering to the biopharmaceutical needs of other establishments as a contract manufacturer.
As a breakthrough player in biologics, To date, the Biopharmaceutical Contract Manufacturing division of Boehringer Ingelheim has actively helped our clients launch scores of biologics in the market that span therapeutic areas such as oncology, immunology, and cardiovascular indications.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
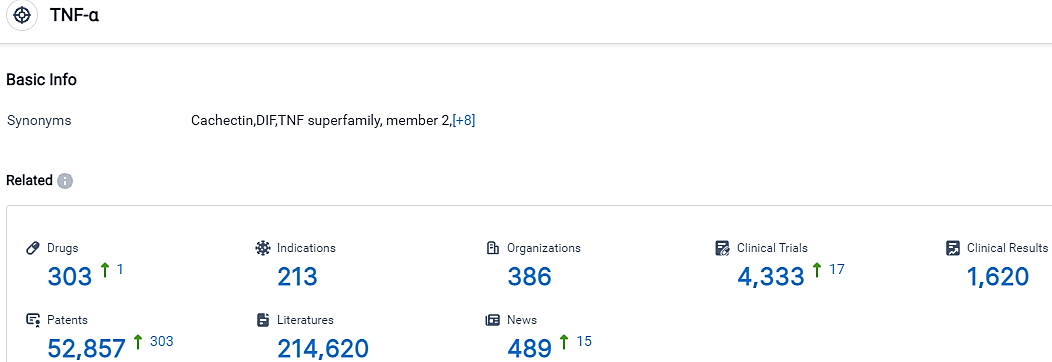
According to the data provided by the Synapse Database, As of October 10, 2023, there are 303 investigational drugs for the TNF-α target, including 213 indications, 386 R&D institutions involved, with related clinical trials reaching 4333,and as many as 52857 patents.
Adalimumab-ADBM is a monoclonal antibody biosimilar drug that targets TNF-α and has been approved for use in various therapeutic areas. Its approval in the United States in 2017 marked an important milestone, providing healthcare professionals and patients with an alternative treatment option for immune-related and inflammatory conditions. Boehringer Ingelheim GmbH, the originator organization, has played a significant role in the development and success of this drug.