Lassen Therapeutics revealed preclinical findings related to LASN01, an Anti-IL-11 Receptor Antibody for Thyroid Eye Disease
Presenting new preclinical data on LASN01 for treating TED, Lassen Therapeutics, a biotechnology firm engaged in clinical stages, is pioneering unique antibody therapeutics. These aim at the interleukin-11 receptor (IL-11R; LASN01) for possible treatment of fibro-inflammatory diseases such as thyroid eye disease and idiopathic pulmonary fibrosis, along with the interleukin-18 binding protein, which may offer a potential remedy for cancer.
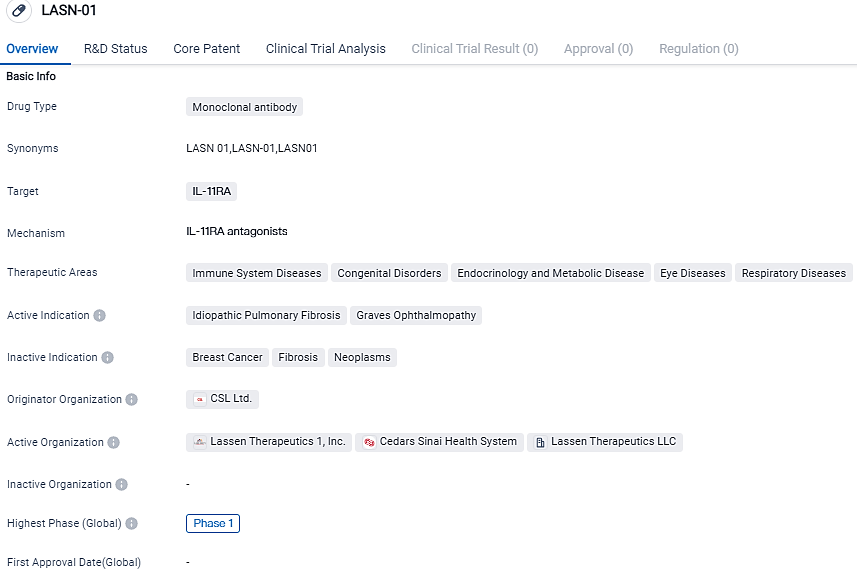
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Our work is focused on showcasing the possible advantages of LASN01 when applied to orbital fibroblasts, which are the relevant cells for thyroid eye disease," stated Lassen CEO Maria Fardis, PhD, MBA. "During our laboratory analyses, LASN01 managed to reduce cell proliferation and the secretion of hyaluronan from the core TED orbital fibroblasts, while also displaying anti-fibrotic properties. This strongly suggests that LASN01 carries the potential to emerge as a new medicinal agent to combat TED."
LASN01 serves as a potent anti-interleukin-11 receptor antibody, which obstructs the pathological pathways of thyroid eye disease that could affect clinical effectiveness. It was demonstrated that LASN01 restricts the release of hyaluronic acid, curtails proliferation, and slows down procollagen synthesis when reacting to IL-11, a combination of IL-11 and IGF-1, or TGFβ in orbital fibroblasts from TED patients post orbital decompression surgery.
The influence that LASN01 has on HA dispatch and OF proliferation was found to be on par with the authorized anti-IGF-1R antibody, teprotumumab, suggesting that LASN01 deserves further exploration as a novel therapeutic tool in the management of TED.
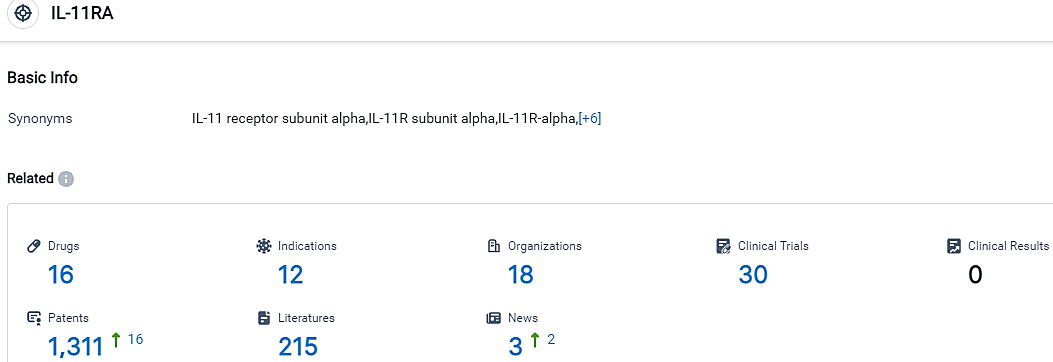
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 10, 2023, there are 16 investigational drugs for the IL-11RA target, including 12 indications, 18 R&D institutions involved, with related clinical trials reaching 30,and as many as 1311 patents.
LASN-01 is a monoclonal antibody drug that targets IL-11RA. It is being investigated for its potential therapeutic benefits in various diseases, including idiopathic pulmonary fibrosis and Graves ophthalmopathy. Currently in Phase 1 of development, LASN-01 holds promise as a potential treatment option in the field of biomedicine.