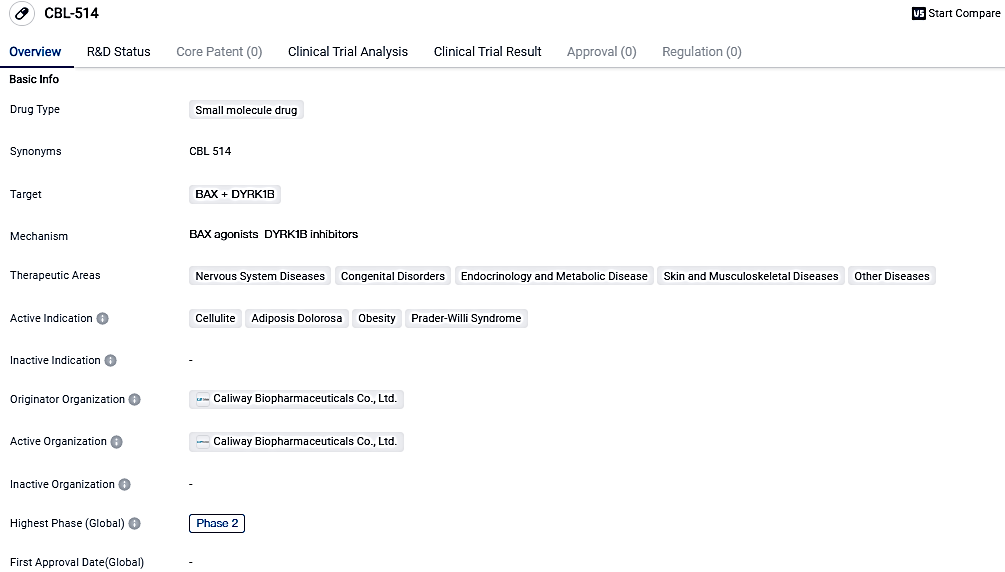
Caliway's CBL-514 demonstrated superior effectiveness compared to Liposuction
Caliway Biopharmaceuticals has communicated the premier outcomes of the phase 2 stage 2 study of CBL-0202, asserting that it has achieved all principal and secondary efficacy endpoints, outperforming placebo in ITT and PP evaluation groups.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"A 2012 research published in the Aesthetic Surgery Journal pointed out that liposuction could eliminate an average of 183.3mL of subcutaneous fat," commented Vivian Ling, Chief Executive Officer of Caliway, "We're pleased to report that CBL-514 has shown better results than liposuction for subcutaneous fat loss in the CBL-0202 stage 2 phase 2 trial, eliminating more than 300mL of subcutaneous fat on average."
The stage 2 of the CBL-0202 study was a randomized, single-blinded, and placebo-controlled phase 2 trial designed to assess the effectiveness, safety, and tolerability of CBL-514, an injection intended for abdominal subcutaneous fat reduction, which was authorized by the US. FDA and Australian Therapeutic Goods Administration. In this process, CBL-514 will be injected into the subcutaneous fat layer of the abdomen.
The trial brought together a randomized group of 76 participants from the United States and Australia, split into a 2:1 ratio to either receive CBL-514 or a placebo. Every participant would then receive up to four treatments of the assigned CBL-514 or placebo, to be administered to the abdomen every four weeks, followed up by two more visits after the final treatment has been administered. Each treatment's highest dosage is 600mg, reliant on the accumulation of subcutaneous fat on each participant's abdomen.
CBL-514, a first-in-class small-molecule pharmaceutical drug, acts as an injection lipolysis medication capable of triggering apoptosis and lipolysis in adipocytes to reduce subcutaneous fat in treated areas, as demonstrated in animal studies, with no systemic impact on the central nervous system, cardiovascular system, or the respiratory system.
Caliway's preclinical studies have demonstrated that CBL-514 reduces the survival kinase DYRK1b, increases caspase 3 and Bax/Bcl-2 ratio apoptosis mediators, and subsequently induces adipocyte apoptosis, both in vivo and in vitro, in a dose-dependent manner.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
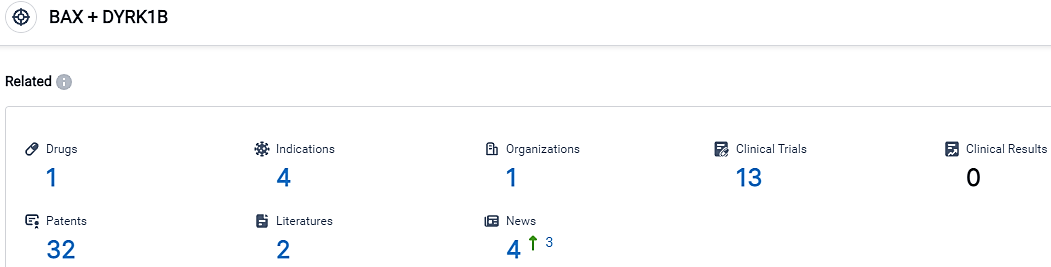
According to the data provided by the Synapse Database, As of October 10, 2023, there are 1 investigational drugs for the BAX and DYRK1B target, including 4 indications, 1 R&D institutions involved, with related clinical trials reaching 13,and as many as 32 patents.
CBL-514 targets BAX and DYRK1B and has shown promise in treating various diseases, including Cellulite, Adiposis Dolorosa, Obesity, and Prader-Willi Syndrome. Caliway is investigating multiple indications for CBL-514, including non-invasive fat reduction, Dercum's disease, cellulite, and lipoma treatment.