Rocket Pharmaceuticals reveals that FDA Acceptance of BLA for RP-L201
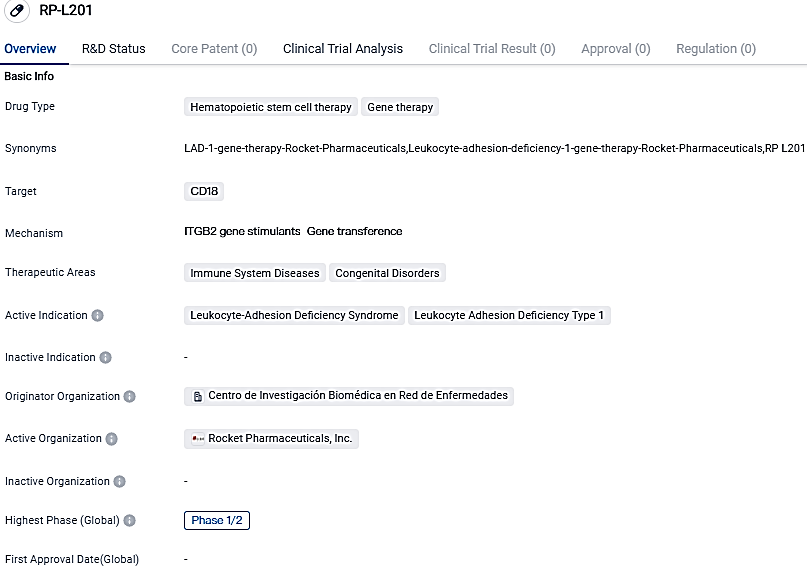
Rocket Pharmaceuticals, Inc., a preeminent biotechnology firm in its late phases, promoting a holistic and enduring line of genetic treatments for rare diseases with significant unmet demand, the U.S. FDA has accepted the BLA and granted Priority Review for RP-L201 (marnetegragene autotemcel), a gene treatment under research, which utilises a lentiviral vector for critical Leukocyte Adhesion Deficiency-I.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The BLA's acceptance by the FDA is a significant stepping stone for Rocket in our bid to provide patients grappling with the severe impacts of LAD-I a one-time gene treatment. Survival past childhood is rare for these patients. Current treatment is limited to bone marrow transplantation, which carries high morbidity and mortality risks and might not be accessible in time for these children," commented Kinnari Patel, Pharm.D., MBA, President and Chief Operating Officer of Rocket Pharma.
Patel added, "We remain profoundly thankful to our patients, caregivers, and researchers for their partnership in this journey. We anticipate maintaining our strong alliance with the FDA during the evaluation phase as we strive to make RP-L201 available to patients in the quickest time frame possible."
Encouraging preliminary data from the worldwide Phase 1/2 trial of RP-L201 showed a 100% survival rate at 12 months post-infusion for all nine LAD-I patients with 12 to 24 months' follow-up data available. It also highlighted significant reductions in the incidences of serious infections compared with patients' pre-treatment histories, improved LAD-I-related skin lesion resolution, and revitalised wound healing capabilities.
Being the Lead Investigator in the U.S, I was responsible for six of the nine LAD-I patients' treatment in this trial. The outcomes are quite extraordinary in my view. Based on my observations, all of them are leading a normal childhood life, which is the ultimate aim of this potentially heal-curing gene treatment," remarked Donald B. Kohn, M.D., an eminent Professor of Microbiology, Immunology & Molecular Genetics, Pediatrics, and Molecular & Medical Pharmacology at University of California.
RP-L201 is an experimental gene treatment that incorporates autologous (patient-derived) hematopoietic stem cells that were genetically altered with a lentiviral vector to introduce a functional copy of the ITGB2 gene. This gene codes for beta-2 integrin component CD18, a crucial protein enabling leukocyte adhesion and their extravasation from blood vessels for infection combat.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
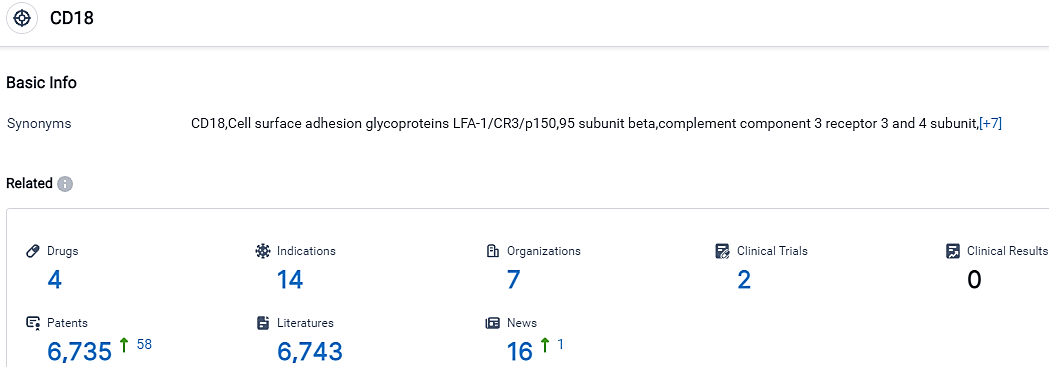
According to the data provided by the Synapse Database, As of October 10, 2023, there are 4 investigational drugs for the CD18 target, including 14 indications, 7 R&D institutions involved, with related clinical trials reaching 2,and as many as 6735 patents.
As RP-L201 progresses through clinical trials, further research and data collection will be necessary to determine its safety, efficacy, and long-term effects. If successful, this drug could provide a much-needed treatment option for patients with LAD and LAD-1, filling a significant unmet medical need in the field of immune system diseases and congenital disorders.