What are FXR agonists and how do you quickly get the latest development progress?
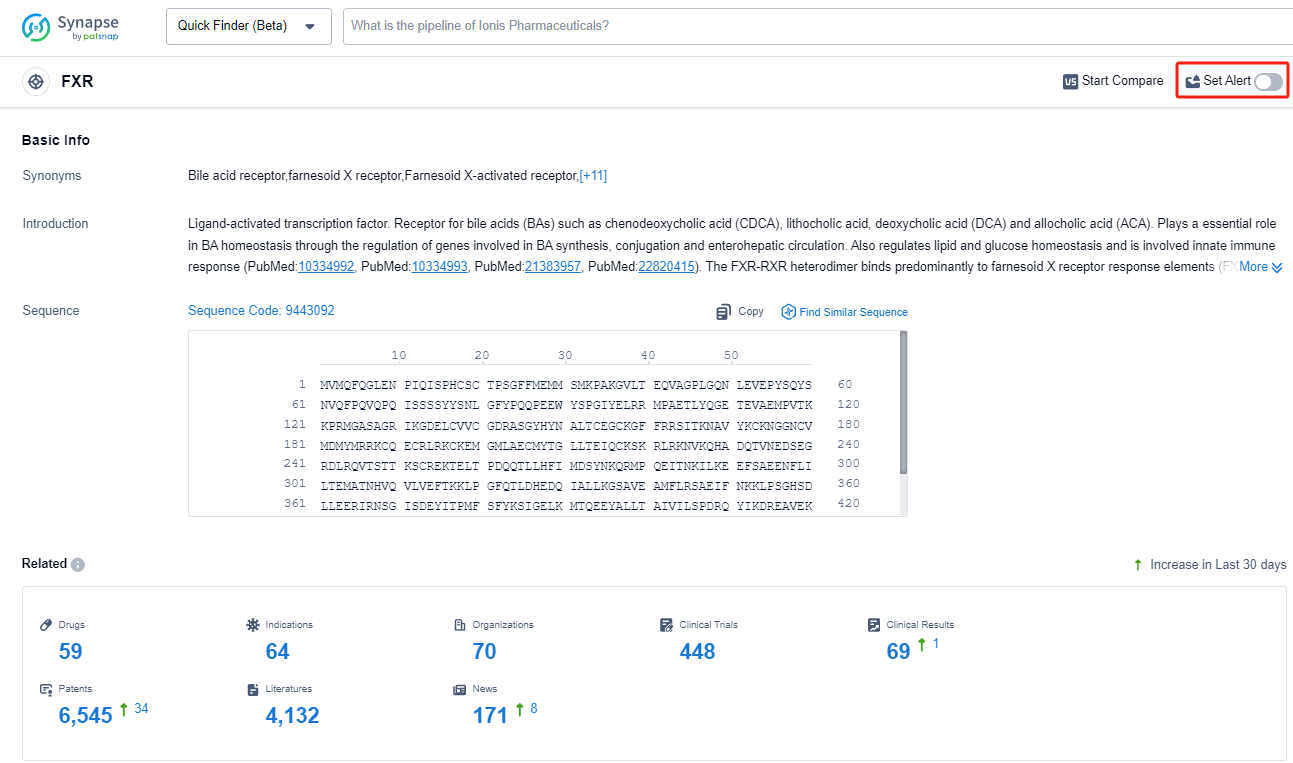
FXR, or farnesoid X receptor, is a crucial nuclear receptor found in the human body. It plays a significant role in regulating bile acid metabolism and maintaining overall liver health. FXR is primarily expressed in the liver, intestine, and kidneys, where it controls the synthesis, transport, and excretion of bile acids. By activating FXR, it promotes the production of bile acids, which aid in the digestion and absorption of dietary fats. Additionally, FXR regulates glucose and lipid metabolism, inflammation, and immune responses. Understanding the role of FXR has opened up new avenues for developing therapeutic interventions targeting liver diseases, metabolic disorders, and gastrointestinal disorders.
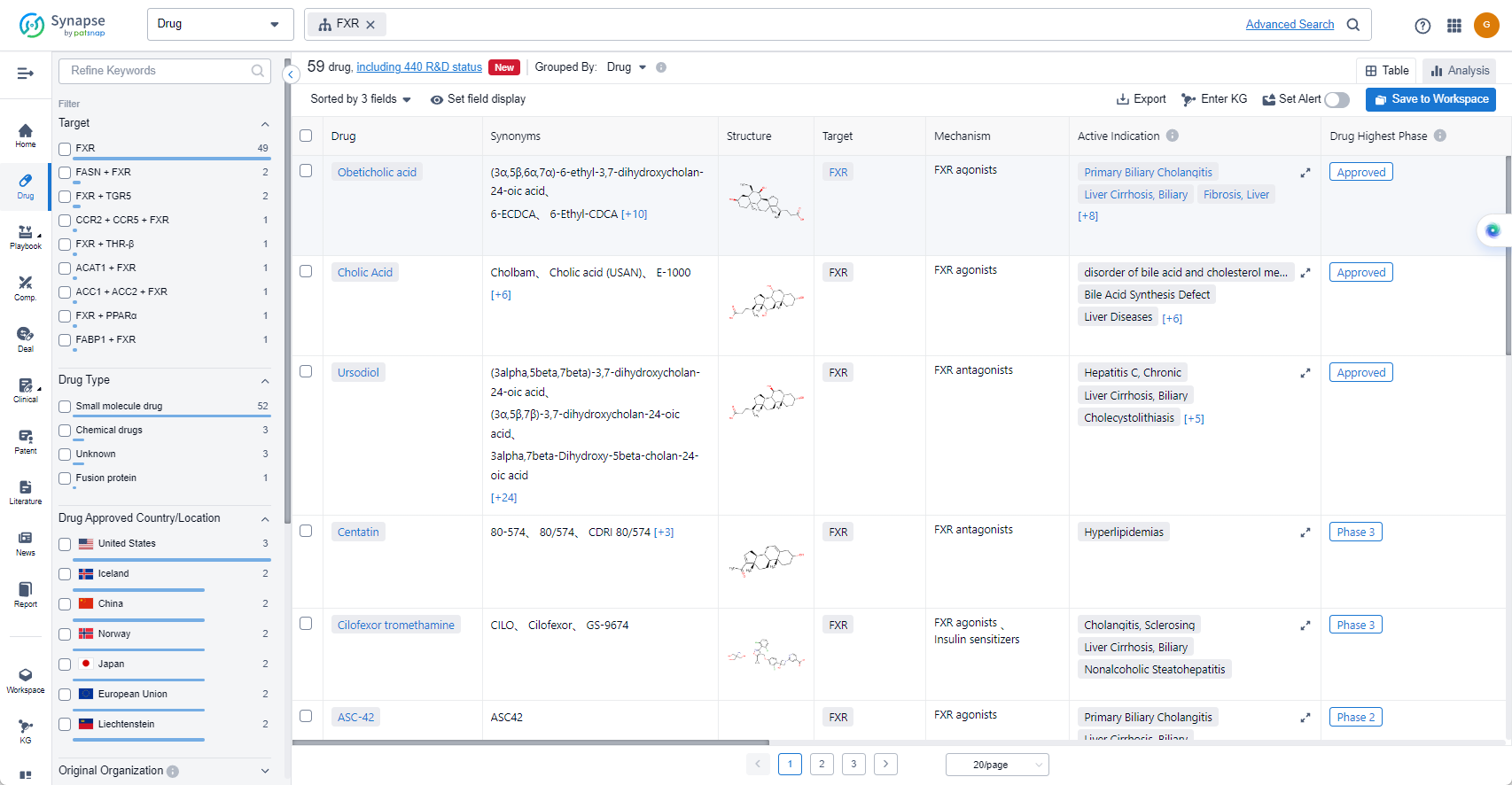
The current competitive landscape of target FXR shows that Intercept Pharmaceuticals, Inc., AbbVie, Inc., and Travere Therapeutics, Inc. are the leading companies with drugs in the highest development phases. These companies have made significant progress in R&D, with drugs in various phases of development.
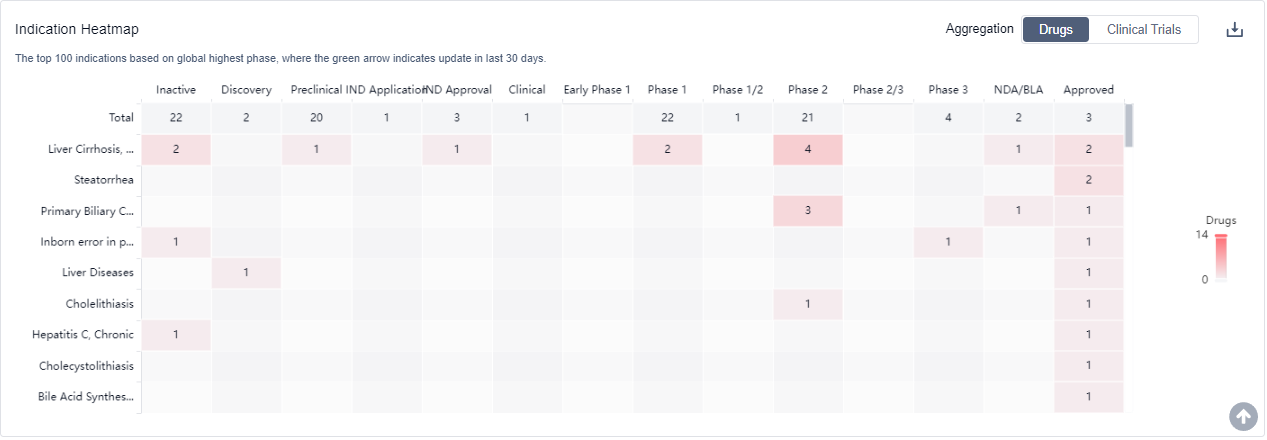
Several drugs under the target FXR have been approved for indications such as Liver Cirrhosis, Biliary; Steatorrhea; Primary Biliary Cholangitis; Inborn error in primary bile acid synthesis; and more.
The most rapidly progressing drug types under the target FXR are Small molecule drugs, Chemical drugs, and Fusion protein. Biosimilars do not pose intense competition in this case.
Countries/locations such as the United States, China, European Union, and Japan are developing rapidly under the target FXR. China has shown progress in the development of drugs under this target.
Overall, the target FXR presents a competitive landscape with multiple companies and drug types in various stages of development. The future development of target FXR holds potential for advancements in the treatment of liver-related indications and offers opportunities for further research and innovation.
How do they work?
From a biomedical perspective, FXR agonists are a type of drug that activate the farnesoid X receptor (FXR) in the body. The FXR is a nuclear receptor that plays a crucial role in regulating bile acid synthesis, transport, and metabolism. By activating the FXR, these agonists can modulate various metabolic pathways related to cholesterol, bile acid, and glucose homeostasis.
FXR agonists have shown potential therapeutic effects in several diseases, particularly in the treatment of liver disorders such as non-alcoholic fatty liver disease (NAFLD) and primary biliary cholangitis (PBC). They can help reduce liver inflammation, improve liver function, and regulate lipid and glucose metabolism.
Furthermore, FXR agonists have also demonstrated promising effects in other conditions like metabolic syndrome, diabetes, and inflammatory bowel disease. They work by influencing gene expression and signaling pathways involved in these diseases.
Overall, FXR agonists are a class of drugs that target the FXR receptor to modulate various metabolic processes, making them a potential therapeutic option for liver and metabolic disorders.
List of FXR Agonists
The currently marketed FXR agonists include:
- Obeticholic acid
- Cholic Acid
- Cilofexor tromethamine
- ASC-42
- Bezafibrate/obeticholic acid
- Cenicriviroc mesylate/Tropifexor
- Cilofexor/Firsocostat
- EDP-305
- HEC-96719
- HPG-1860
For more information, please click on the image below.
What are FXR agonists used for?
FXR agonists are commonly used in the treatment of liver disorders such as non-alcoholic fatty liver disease (NAFLD) and primary biliary cholangitis (PBC). For more information, please click on the image below to log in and search.
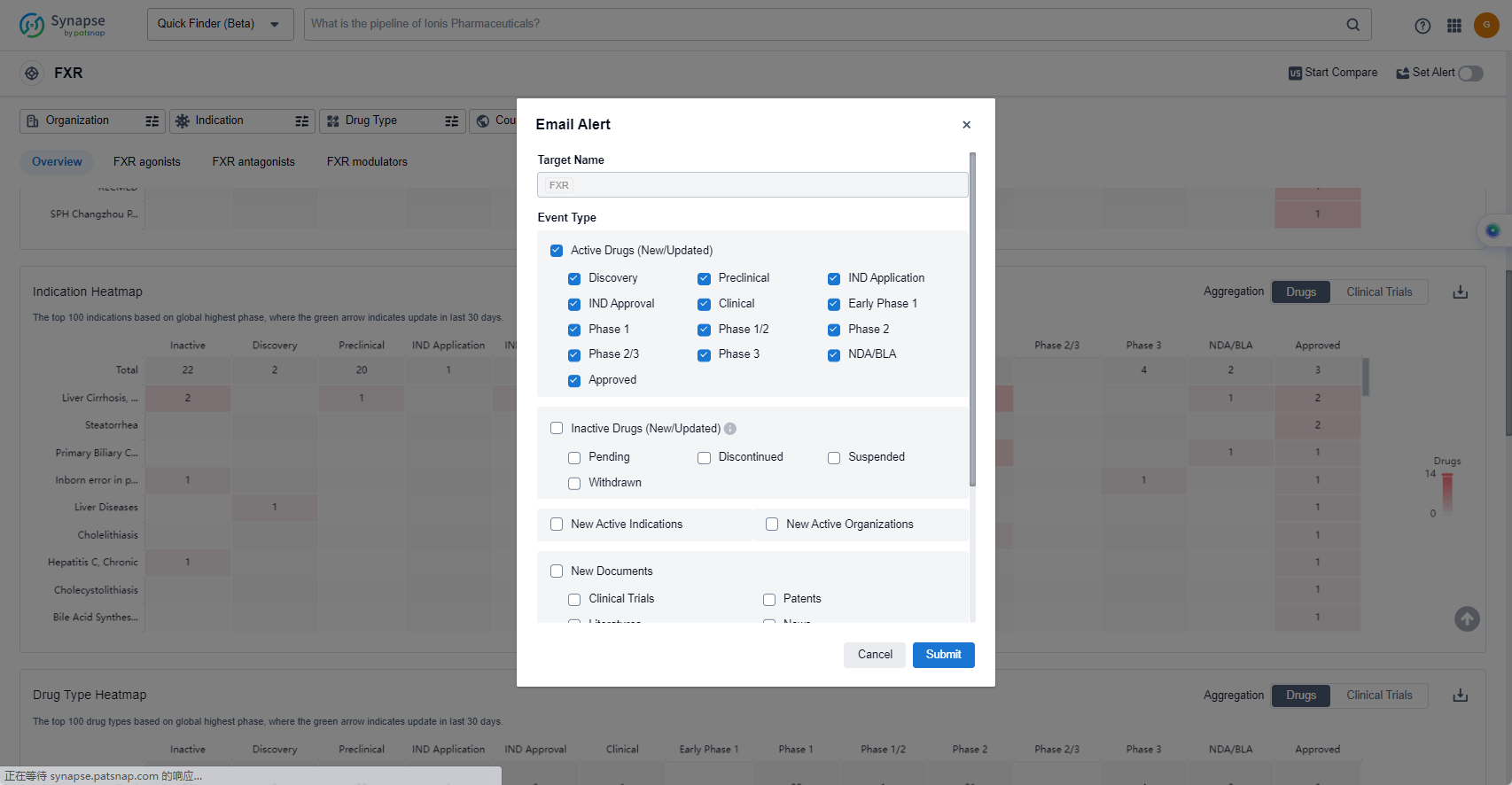
How to obtain the latest development progress of FXR agonists?
In the Synapse database, you can keep abreast of the latest research and development advances of FXR agonists anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!