Breakthrough Pain Relief Innovation: Patent Exploration of Next-Generation NaV1.8 Inhibitors
In clinical practice, pain is a frequently encountered symptom. According to a report by Mordor Intelligence, the global pain management market was valued at $79.4 billion in 2021 and is projected to grow to $120.7 billion by 2027, with a compound annual growth rate (CAGR) of 7.39%. Consequently, the market for analgesics is seen as another billion-dollar opportunity following GLP-1 weight loss drugs. Currently, pain relief drugs are primarily composed of antipyretic analgesics/non-steroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics. While NSAIDs are considered safe, their analgesic efficacy is relatively weak. Conversely, opioid drugs such as morphine and hydromorphone offer potent pain relief but are associated with significant addiction risks and widespread misuse. This has created an immense and urgent demand for novel pain relief medications.
In July of this year, Vertex announced that the FDA accepted its new pain medication VX-548 (Suzetrigine) for the treatment of moderate to severe acute pain and granted it priority review. The safety and efficacy of Suzetrigine make it suitable for managing moderate to severe acute pain. If approved, it would become the world’s first non-opioid, non-addictive pain treatment to be launched in over two decades, marking a groundbreaking advancement.
VX-548 is an oral selective NaV1.8 inhibitor that demonstrates high selectivity for NaV1.8 compared to other NaV ion channels. NaV1.8 plays a critical role in pain signal transmission within the peripheral nervous system. By blocking the NaV1.8 channel, these inhibitors prevent pain signals from traveling from the peripheral to the central nervous system, thereby providing pain relief. Since NaV1.8 is predominantly expressed in nociceptive sensory neurons and does not participate in central nervous system functions, NaV1.8 inhibitors are less likely to cause the common side effects associated with non-selective NaV channel inhibitors. More importantly, NaV1.8 inhibitors do not exhibit the addictive properties of opioids and do not negatively impact motor, cognitive, or memory functions. These characteristics make NaV1.8 inhibitors a highly promising focus in the development of next-generation pain therapies.
The importance of NaV1.8 as a target in pain relief has made it a hot area in pharmaceutical research and development, prompting major pharmaceutical companies to pursue patent strategies in this domain. Using VX-548 as a starting point, we can explore fast-followers in this space and examine the patent landscape for NaV1.8 inhibitors among various pharmaceutical companies.
To do this, we can utilize Patsnap Chemical. By selecting structure-based search, we can input VX-548’s common identifiers, such as CAS number, generic name, molecular formula, or SMILES file. Alternatively, we can directly edit the structure in the drawing tool or upload a ChemDraw structure file in MOL format. In this case, we use similarity search (setting the Tanimoto coefficient to 0.7) to identify patents related to structures similar to VX-548.
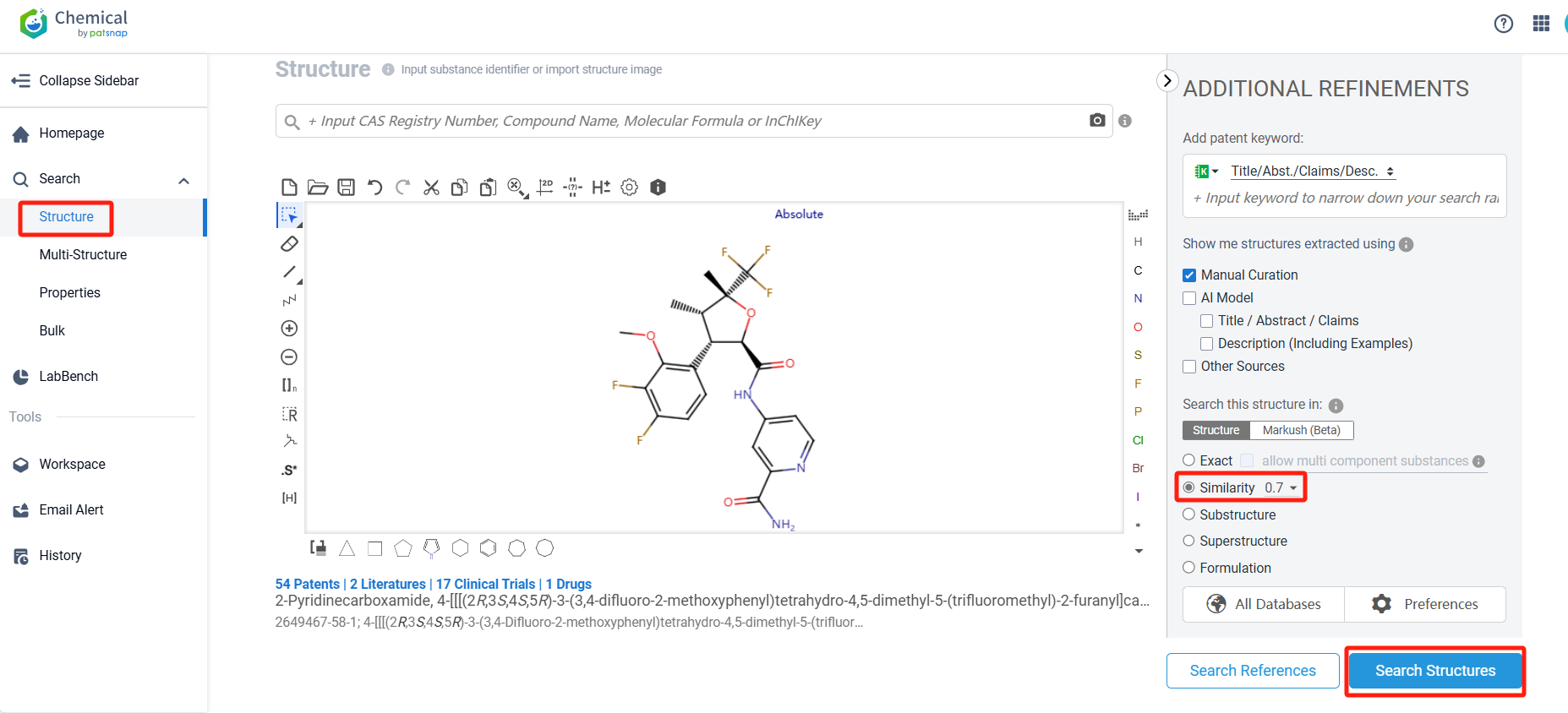
After searching for compounds, we can identify 47 patents related to VX-548 with structurally similar compounds, along with relevant literature and other compounds. To facilitate patent analysis, we can click the "View in Analytics" link to access Patsnap Analytics.
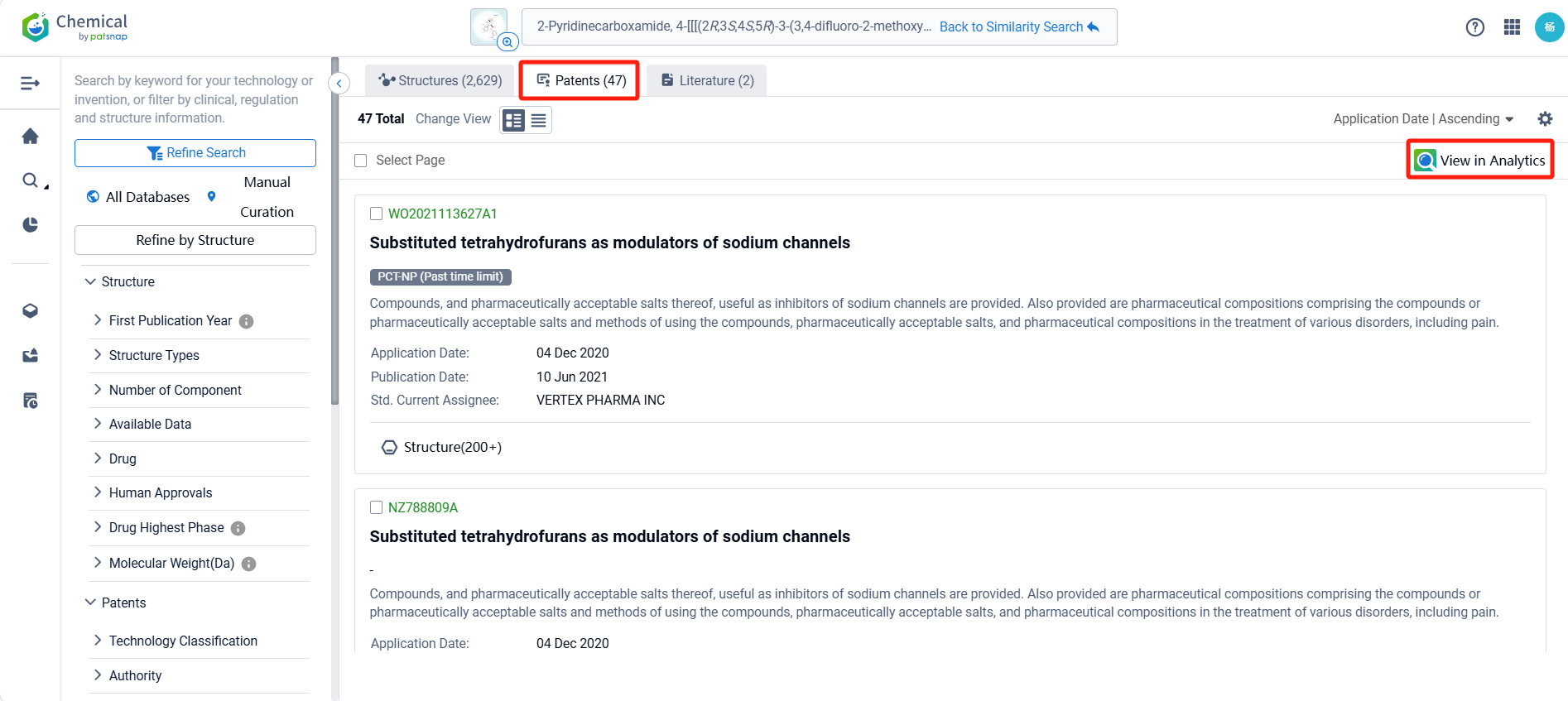
Once linked to Patsnap Analytics, sorting patents by application date reveals that VX-548 was first disclosed in Vertex’s PCT international application WO2021113627A1 (application date: December 4, 2020; publication date: June 10, 2021). This patent has been granted in the U.S., Europe, and China.
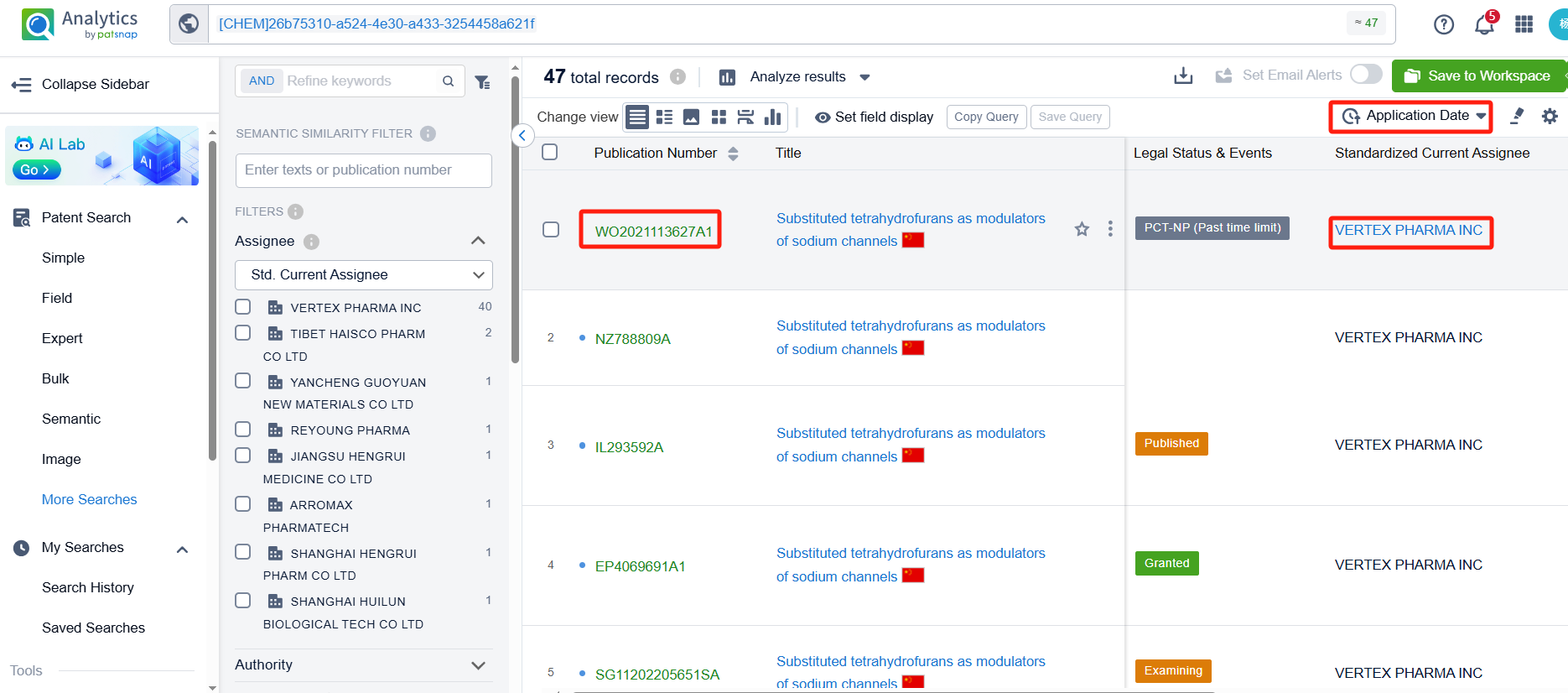
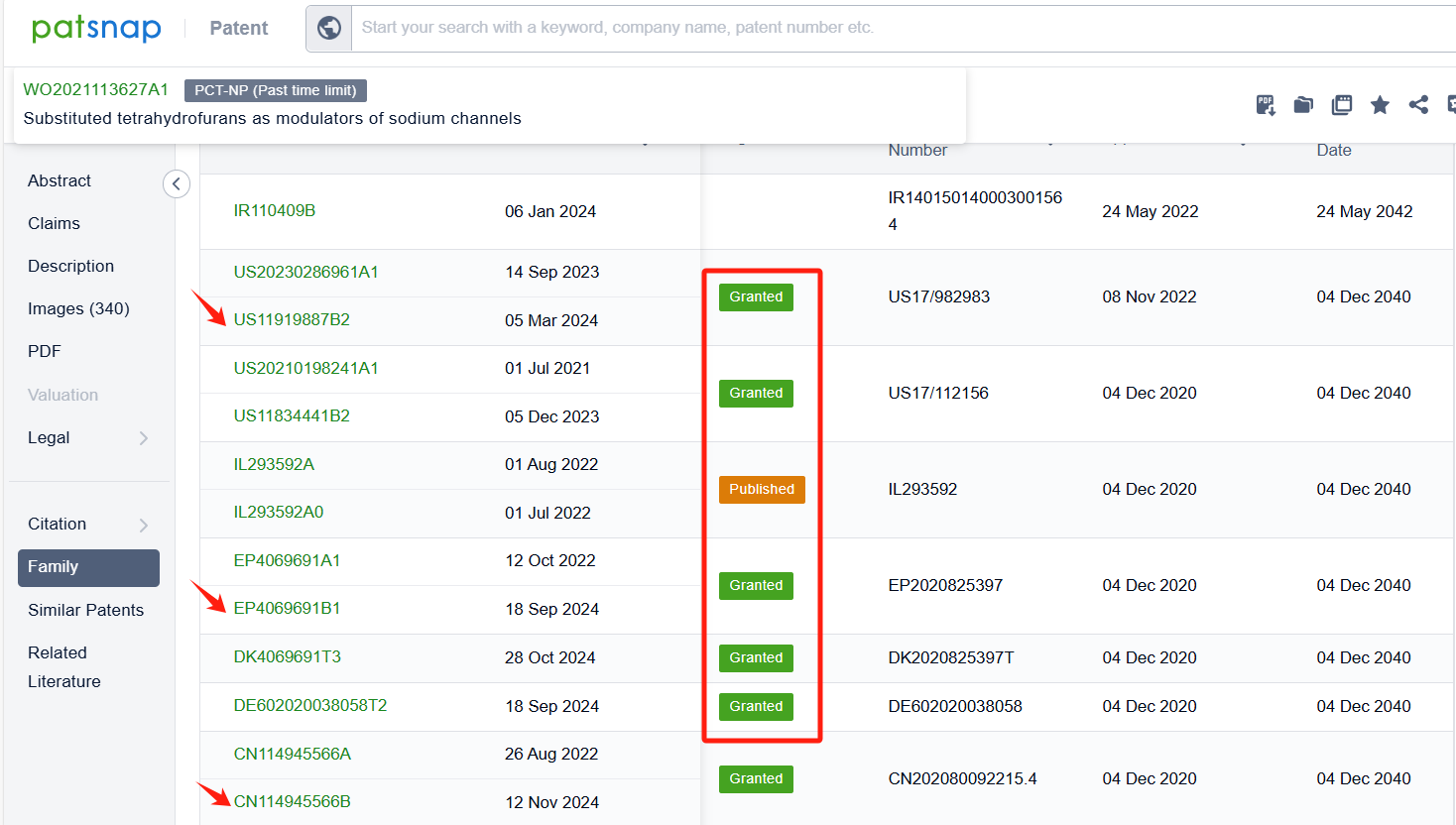
To focus on competitor patents, we filter out Vertex. After exclusion, 7 additional patents remain. By clicking the patent numbers, detailed patent information can be reviewed.
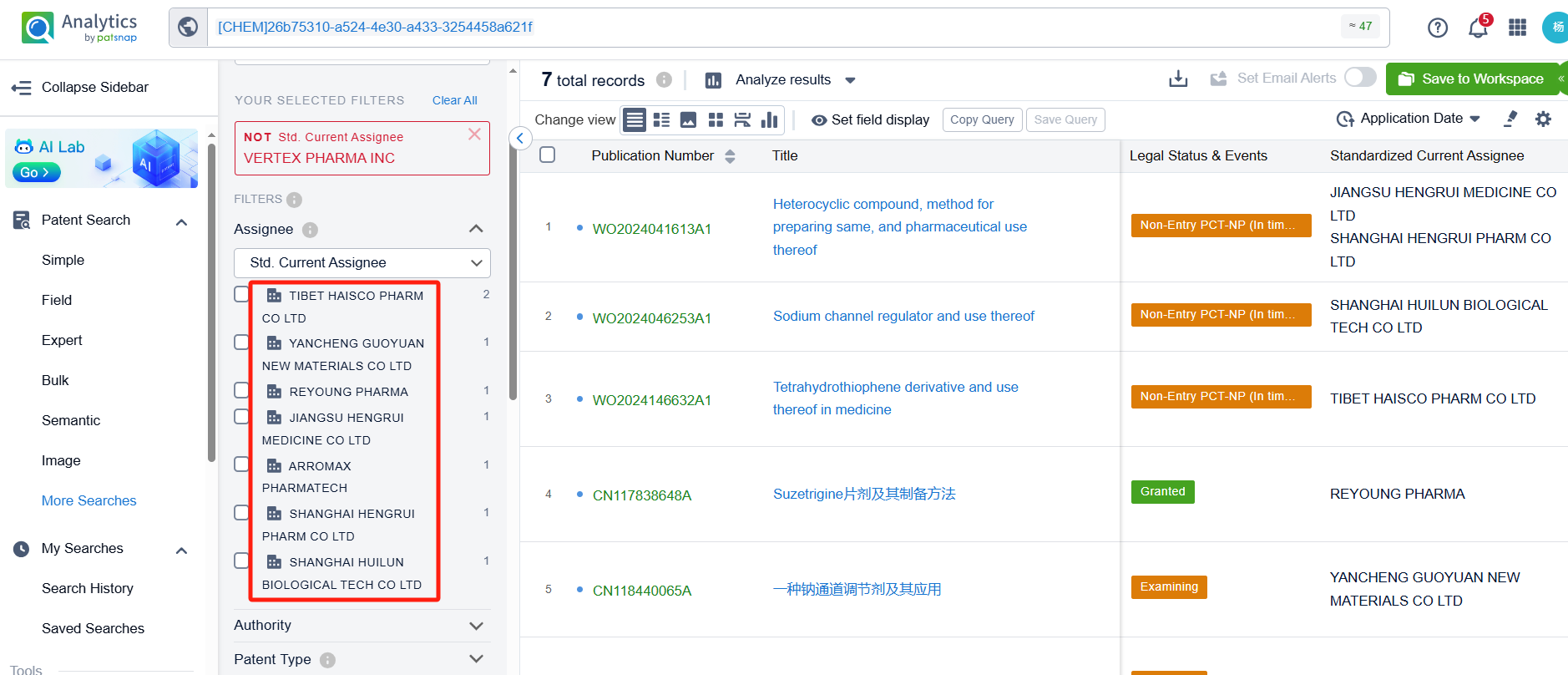
The patents for compounds similar to VX-548 involve companies such as Haisco Pharmaceutical, Yancheng Guoyuan New Materials, Hengrui Pharmaceutical, Anrun Pharmaceutical, and Shanghai Huilun Life Science. A detailed structural analysis reveals several noteworthy strategies:
Hengrui Pharmaceutical:
Patent WO2024041613A1 (application date: August 24, 2023; publication date: February 29, 2024) modified VX-548’s pyridine side chain, replacing a formamide group with an amidine. This pyridine side chain, critical for molecular solubility, suggests potential pharmacokinetic improvements. Experimental results show compounds 16 and 32 exhibit strong NaV1.8 inhibition and favorable pharmacokinetics.
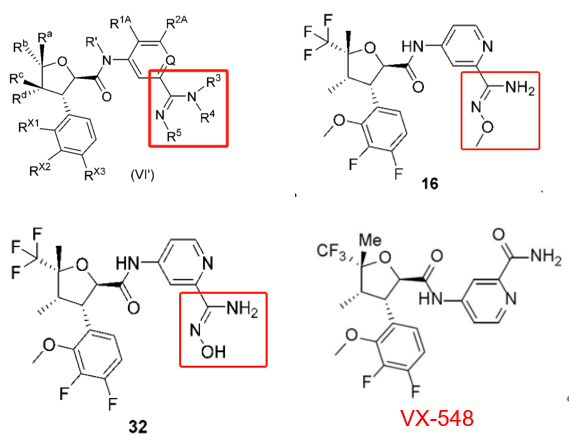
Shanghai Huilun Life Science:
Patent WO2024046253A1 (application date: August 28, 2023; publication date: March 7, 2024) made broader modifications. Alterations include fused bicyclic structures in ring A, replacing the furan ring with a sulfonamide. Experimental results indicate higher pain thresholds compared to VX-548, which may enhance the patent’s claim protection.
Haisco Pharmaceutical:
Patent WO2024146632A1 (application date: August 14, 2023; publication date: January 5, 2024) replaced the tetrahydrofuran structure in VX-548 with tetrahydrothiophene, a bioisostere. However, this change might struggle to overcome inventive step barriers, as suggested by the inclusion of eight X references in the international search report.
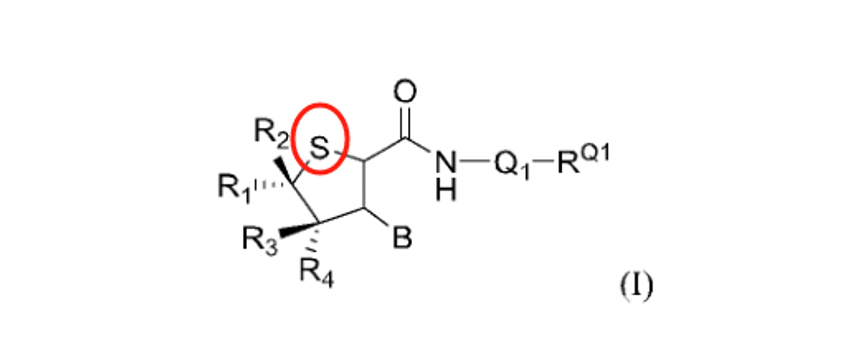
Another patent, WO2024188367A1 (application date: May 8, 2024; publication date: September 19, 2024), covers compounds with oxygen in the amide bond replaced by sulfur. Experimental results highlight promising pharmacokinetics, crucial for its approval.
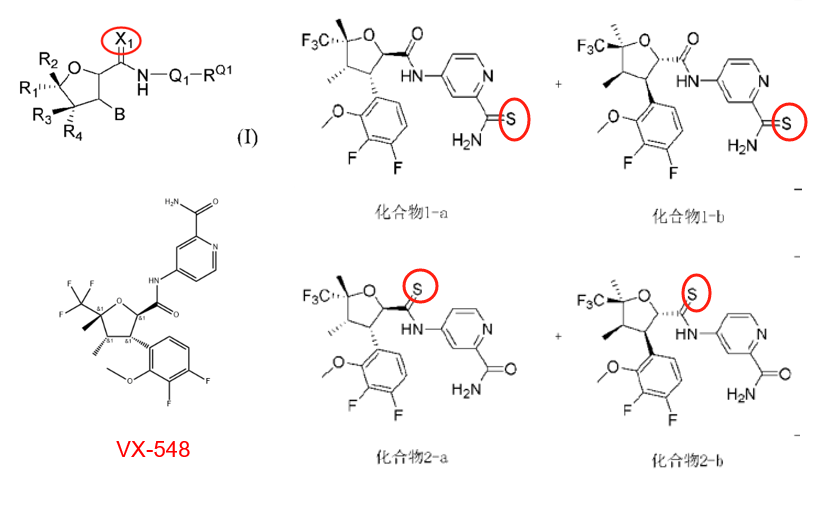
Anrun Pharmaceutical:
Patent CN118388466A (application date: April 23, 2024; publication date: July 26, 2024) modified the phenyl side chain by replacing methoxy with difluoromethoxy. This change reportedly enhances NaV1.8 inhibition and pharmacokinetics.
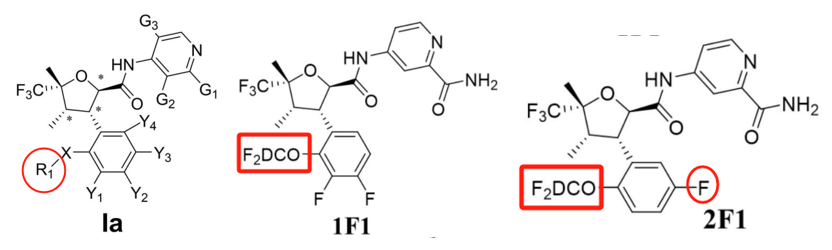
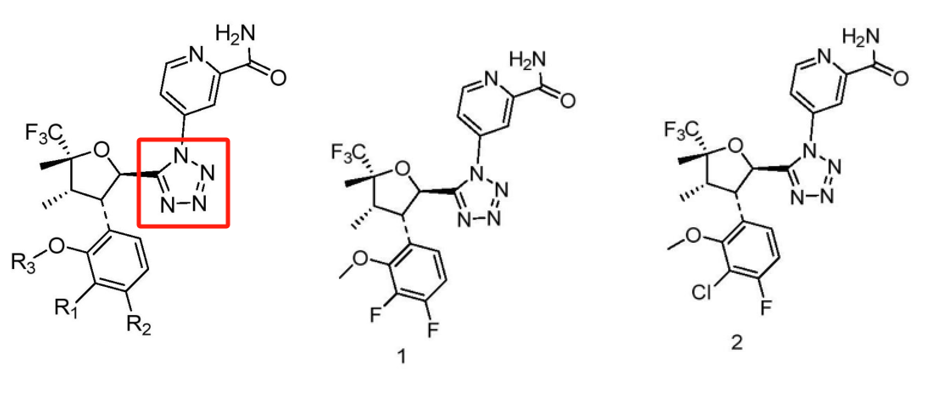
Yancheng Guoyuan New Materials:
Patent CN118440065A (application date: April 18, 2024; publication date: August 6, 2024) replaced the amide linker with a tetrazole group. Comparative experiments with VX-548 show improved NaV1.8 inhibition.
Hengrui’s WO2024041613A1 is notable as the earliest fast-follower, filed just 14 months after VX-548’s publication. Shanghai Huilun Life Science’s WO2024046253A1 was also filed around the same time. Both companies targeted the pyridine ring's formamide group but diverged in modification approaches. Other companies’ core structural innovations, while incremental, show significant improvements in activity or pharmacokinetics.
As a first-in-class drug, VX-548 has remarkable commercial value. The NaV1.8 inhibitor space is rapidly evolving, with multiple companies advancing drug development and clinical trials. The growing competition will likely yield more potent and versatile NaV1.8 inhibitors, providing expanded treatment options for patients suffering from pain.
The fierce R&D competition underscores the importance of Freedom-to-Operate (FTO) analysis. Tools like Patsnap Chemical streamline FTO assessments by linking chemical structures to patents, literature, and clinical trial data. This integration supports strategic decision-making in new drug development and intellectual property protection.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.




