European Commission Approves AbbVie's ELAHERE® for Platinum-Resistant Ovarian Cancer
AbbVie (NYSE: ABBV) has announced that the European Commission (EC) has approved ELAHERE® (mirvetuximab soravtansine) for marketing. This treatment is intended for adult patients with folate receptor-alpha (FRα) positive, platinum-resistant high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who have previously undergone one to three systemic therapy regimens. ELAHERE is recognized as the first and only folate receptor alpha (FRα)-targeted antibody-drug conjugate (ADC) to receive approval in the European Union (EU), as well as in Iceland, Liechtenstein, Norway, and Northern Ireland.
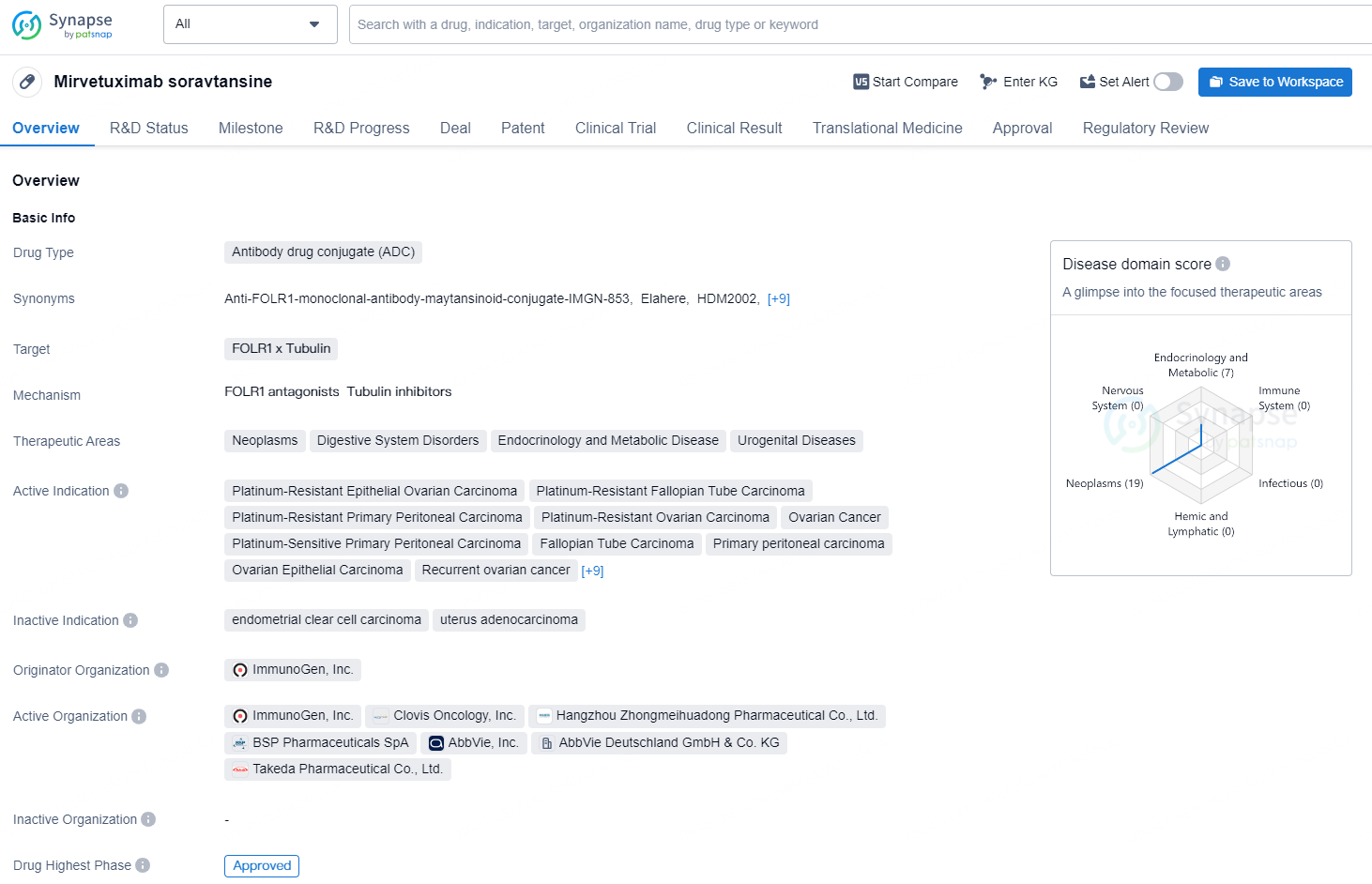
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"It's been a decade since the last treatment for platinum-resistant ovarian cancer was sanctioned in the EU, and now healthcare professionals have a new, effective targeted therapy available for these patients," stated Toon Van Gorp, Professor of Gynaecological Oncology at the University of Leuven.
Ovarian cancer ranks among the top contributors to mortality from gynecological cancers worldwide. Most patients are diagnosed with advanced-stage disease and usually receive surgical intervention followed by platinum-based chemotherapy. However, a significant number eventually develop platinum-resistant disease. Traditionally, the treatment landscape for those with platinum-resistant ovarian cancer (PROC) has been rather constrained, and existing therapies often lead to side effects that can adversely affect patients' quality of life.
"Ovarian cancer can be incredibly damaging, taking women away from cherished moments with their loved ones, disrupting their professional lives, and hindering their valuable roles in society," remarked Clara Mackay, CEO of the World Ovarian Cancer Coalition. "In Europe, ovarian cancer is three times more lethal than breast cancer, and the availability of innovative treatment options moves us closer to a future where everyone diagnosed with ovarian cancer can achieve the highest possible survival rates and quality of life, regardless of their location."
Approximately one-third of individuals diagnosed with ovarian cancer exhibit high expression of the folate-receptor alpha (FRα) biomarker (≥75% of tumor cells with ≥2+ membrane staining intensity). To assess biomarker status, patients may be evaluated using Roche’s VENTANA® FOLR1 (FOLR1-2.1) RxDx Assay either at the time of diagnosis or upon the initial indication of resistance to platinum-based therapies. AbbVie worked in partnership with Roche Diagnostics to develop the newly approved immunohistochemistry (IHC) companion diagnostic test designed to identify candidates who may benefit from ELAHERE.
"The European Commission's approval of ELAHERE offers a critically needed, clinically significant option for patients who receive the devastating news of their ovarian cancer recurrence and who are anxious about their subsequent treatment options after developing platinum-resistance," explained Roopal Thakkar, M.D., executive vice president, research and development, and chief scientific officer at AbbVie.
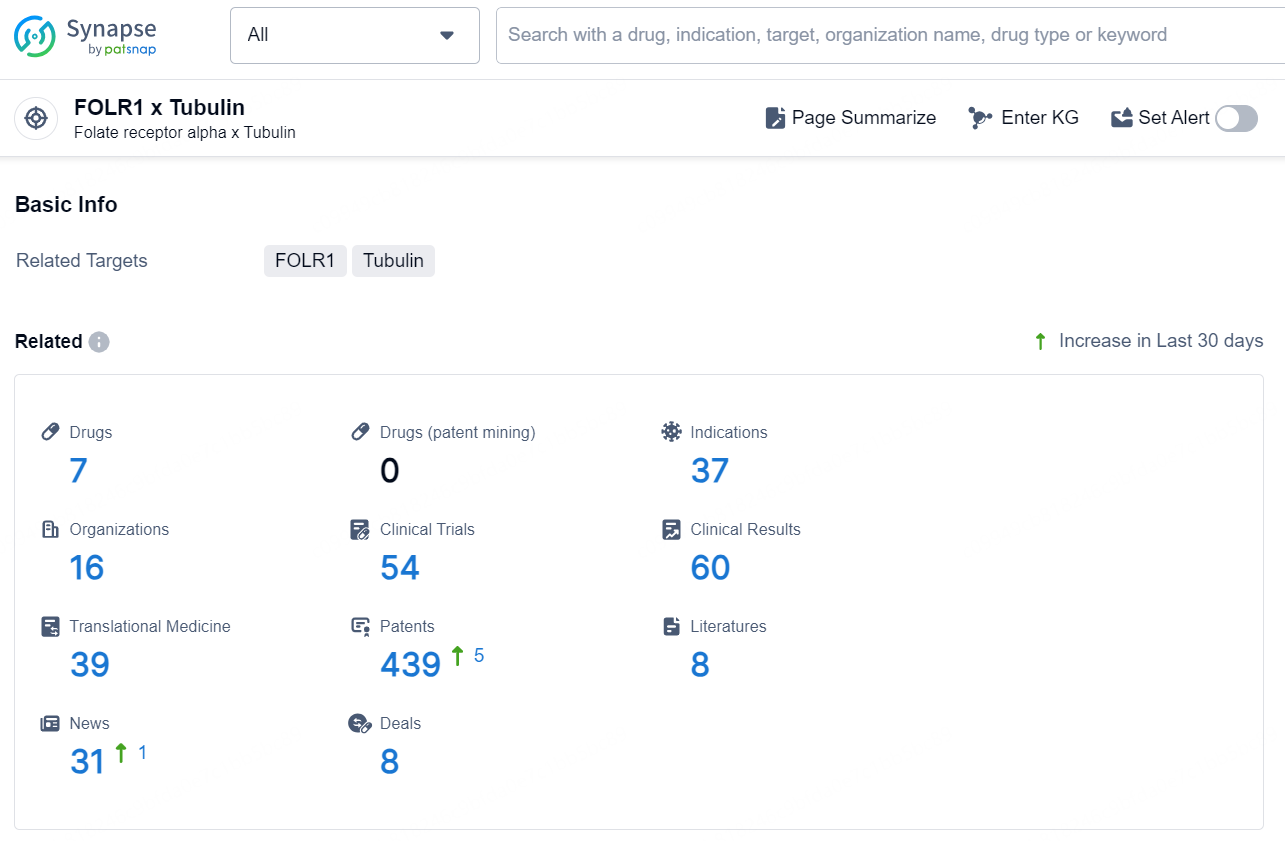
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 7 investigational drugs for the FOLR1 x Tubulin target, including 37 indications, 16 R&D institutions involved, with related clinical trials reaching 54, and as many as439 patents.
Mirvetuximab soravtansine is an antibody drug conjugate (ADC) that targets FOLR1 and tubulin. Mirvetuximab soravtansine (approved under the brand name ELAHERE®) was granted approval by the European Commission in November 2024, and was granted full FDA approval in the United States in March 2024. Marketing authorization submissions for mirvetuximab soravtansine are under review in multiple other countries.