bupivacaine hydrochloride Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Bupivacaine hydrochloride's R&D Progress
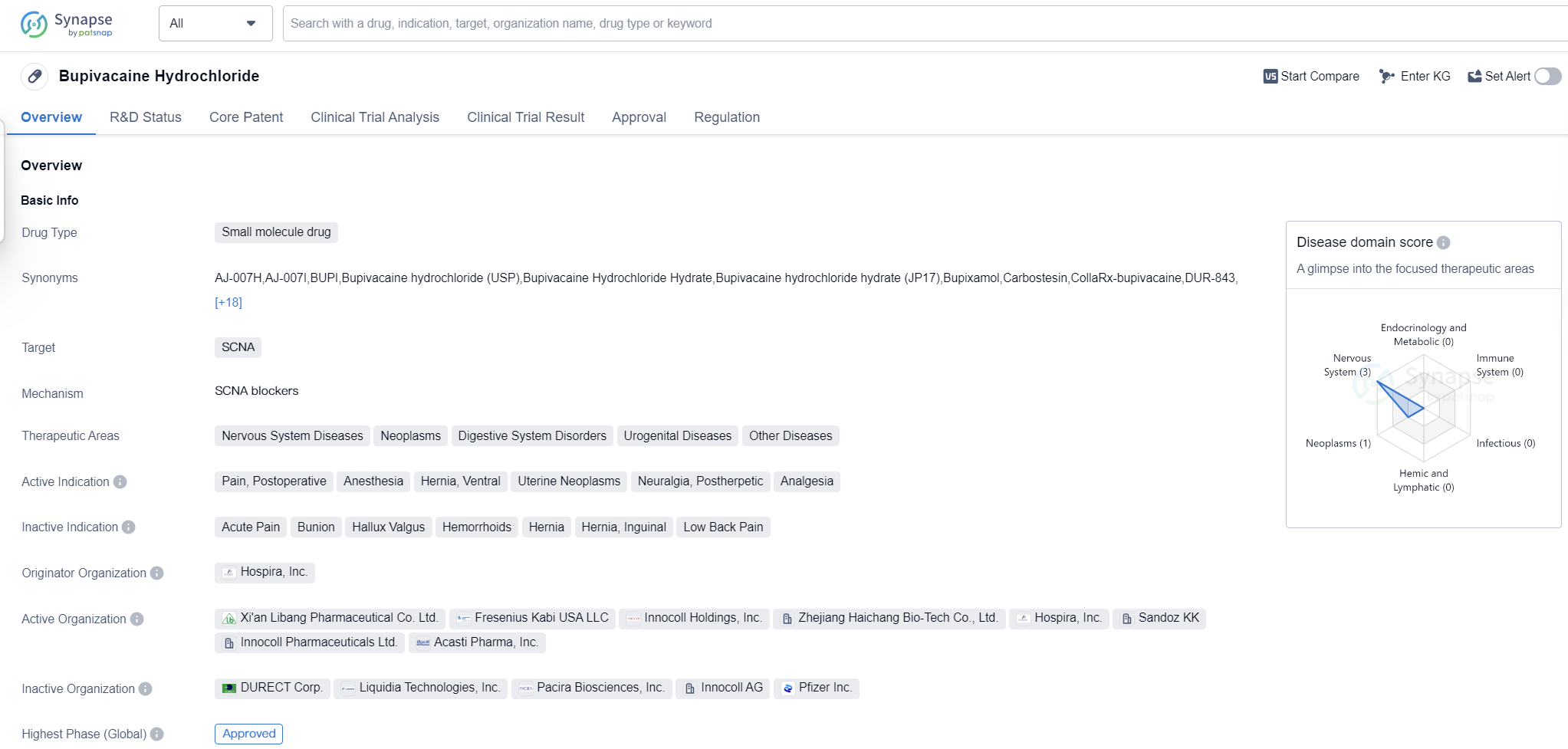
Bupivacaine Hydrochloride is a small molecule drug that primarily targets the SCNA (sodium channel, voltage-gated, type V, alpha subunit) protein. It is used in the treatment of various therapeutic areas including Nervous System Diseases, Neoplasms, Digestive System Disorders, Urogenital Diseases, and Other Diseases. The drug is indicated for the management of pain, postoperative pain, anesthesia, hernia, ventral, uterine neoplasms, neuralgia, postherpetic, and analgesia.
The drug was developed by Hospira, Inc., a pharmaceutical company. It received its first approval in Japan in September 1969, making it one of the earliest approved drugs in the market. Bupivacaine The highest R&D phase of this drug is approved.
In terms of regulatory status, Bupivacaine Hydrochloride is classified as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This classification often provides certain incentives and benefits to the manufacturer, such as extended market exclusivity and financial support for research and development.
The drug's primary mechanism of action involves blocking the sodium channels, thereby inhibiting the transmission of pain signals. This makes it an effective option for pain management in various clinical settings, including surgical procedures and chronic pain conditions.
Bupivacaine Hydrochloride's approval in global markets highlights its widespread use and recognition in the medical community. Its long history of use and established safety profile make it a trusted option for healthcare professionals.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for bupivacaine hydrochloride: SCNA blockers
SCNA blockers are a type of drug that act as blockers for sodium channels. Sodium channels are proteins found in cell membranes that play a crucial role in the transmission of electrical signals in cells. They are particularly important in nerve cells, where they help generate and propagate action potentials.
SCNA blockers work by binding to sodium channels and preventing them from opening or functioning properly. This inhibition of sodium channel activity can have various effects depending on the specific drug and its target. In the context of biomedicine, SCNA blockers are often used as medications to treat conditions such as epilepsy, neuropathic pain, and cardiac arrhythmias.
By blocking sodium channels, SCNA blockers can reduce the excessive firing of neurons, thereby preventing seizures in epilepsy patients. They can also inhibit the transmission of pain signals in the nervous system, providing relief for individuals suffering from neuropathic pain. In the case of cardiac arrhythmias, SCNA blockers can help regulate the electrical activity of the heart and restore normal rhythm.
Overall, SCNA blockers are an important class of drugs that target sodium channels to modulate electrical signaling in cells. Their use in biomedicine has significant therapeutic implications for various conditions related to abnormal electrical activity in the nervous system and the heart.
Drug Target R&D Trends for bupivacaine hydrochloride
SCNAs, or Sodium Channel Navs, play a crucial role in the human body by facilitating the movement of sodium ions across cell membranes. These channels are responsible for generating and propagating electrical signals in various tissues, including the brain, heart, and muscles. By controlling the flow of sodium ions, SCNAs regulate the excitability and function of cells, enabling processes such as nerve transmission, muscle contraction, and cardiac rhythm. Dysregulation or mutations in SCNAs can lead to various disorders, including epilepsy, cardiac arrhythmias, and muscle disorders. Understanding the role of SCNAs is essential for developing targeted therapies and improving treatments for these conditions.
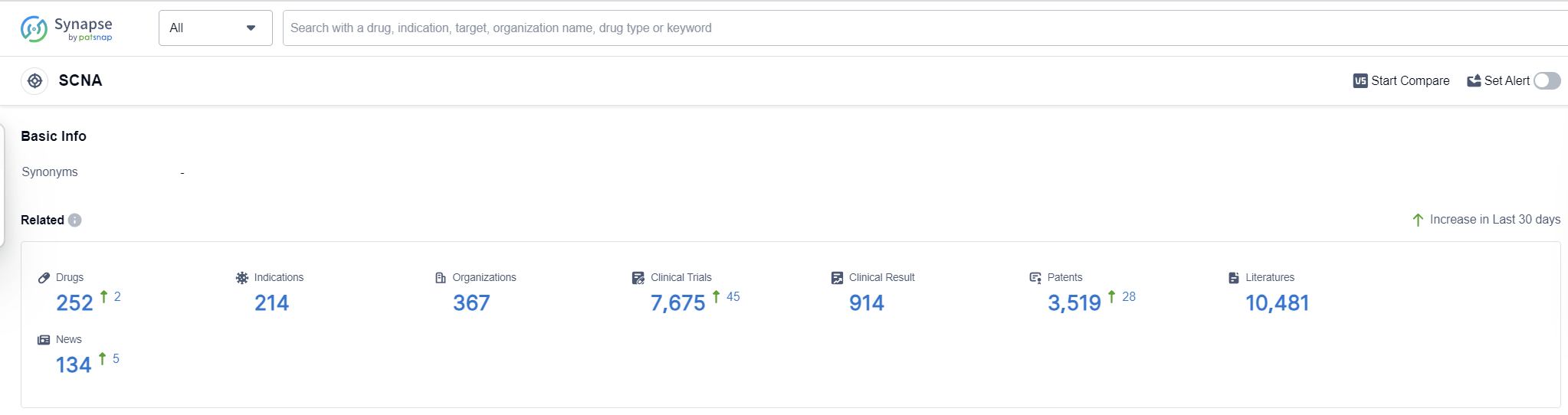
According to Patsnap Synapse, as of 16 Sep 2023, there are a total of 252 SCNA drugs worldwide, from 367 organizations, covering 214 indications, and conducting 7675 clinical trials.
The analysis of the target SCNA reveals a competitive landscape with several companies actively developing drugs. Novartis AG and Pfizer Inc. emerge as key players with a high number of approved drugs and diverse portfolios. The focus on indications such as anesthesia, arrhythmias, cardiac, and pain highlights the therapeutic areas where significant progress has been made. Small molecule drugs dominate the development phase, indicating their importance in addressing the target SCNA. China, the United States, Japan, and the European Union are leading in terms of drug development, with China showing remarkable progress. Overall, the target SCNA presents opportunities for innovation and competition in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Bupivacaine Hydrochloride is a small molecule drug developed by Hospira, Inc. It targets the SCNA protein and is indicated for the treatment of various diseases, primarily related to the nervous system. With its approval in multiple countries and its classification as an orphan drug, Bupivacaine Hydrochloride has proven to be a valuable option for pain management in different clinical settings.