Pharmaceutical Insights: alverine citrate's R&D Progress and its Mechanism of Action on Drug Target
Alverine citrate's R&D Progress
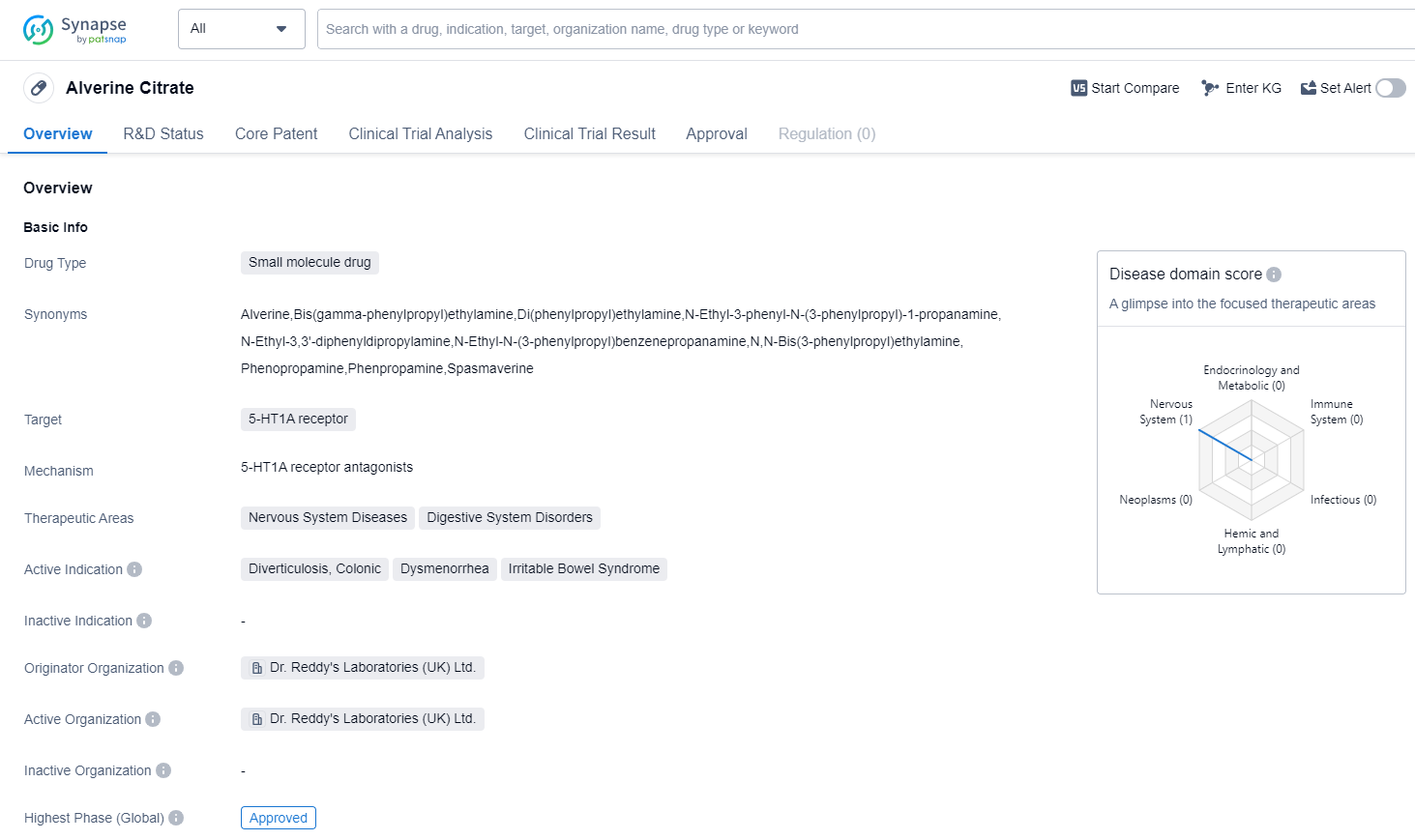
Alverine Citrate is a small molecule drug that targets the 5-HT1A receptor. It is primarily used in the treatment of nervous system diseases and digestive system disorders. The drug has been approved for the treatment of diverticulosis, colonic, dysmenorrhea, and irritable bowel syndrome.
The originator organization of Alverine Citrate is Dr. Reddy's Laboratories (UK) Ltd. This pharmaceutical company is responsible for the development and production of the drug. The highest phase of development for Alverine Citrate is approved, indicating that it has successfully completed clinical trials and has been granted regulatory approval for use.
The first approval of Alverine Citrate occurred in September 2018 in the United Kingdom. This means that it was the first country/location to grant regulatory approval for the drug. The approval in the UK signifies that the drug has met the necessary safety and efficacy requirements set by the regulatory authorities in that country.
Alverine Citrate is a promising drug in the field of biomedicine, specifically in the treatment of nervous system diseases and digestive system disorders. Its approval for the treatment of diverticulosis, colonic, dysmenorrhea, and irritable bowel syndrome highlights its potential therapeutic benefits in these conditions.
As a small molecule drug, Alverine Citrate is likely to have a well-defined chemical structure and mechanism of action. This can facilitate its production and formulation into various dosage forms for patient use. The targeting of the 5-HT1A receptor suggests that the drug may modulate serotonin signaling, which is involved in the regulation of various physiological processes.
The approval of Alverine Citrate in the UK is a significant milestone for Dr. Reddy's Laboratories (UK) Ltd. It demonstrates their ability to successfully develop and bring a novel drug to market. The approval also opens up opportunities for the company to expand its market presence and potentially seek regulatory approvals in other countries.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for alverine citrate: 5-HT1A receptor
5-HT1A receptor antagonists are a type of drug that specifically target and block the 5-HT1A receptors in the brain. These receptors are a subtype of serotonin receptors, which are involved in the regulation of various physiological processes and neurotransmission. By antagonizing or blocking the 5-HT1A receptors, these drugs can modulate the activity of serotonin, a neurotransmitter that plays a role in mood, anxiety, and other mental processes.
From a biomedical perspective, 5-HT1A receptor antagonists are used in the treatment of various psychiatric and neurological disorders. For example, they are commonly prescribed as antidepressants, as they can increase the levels of serotonin in the brain, which is often associated with improved mood and decreased depressive symptoms. Additionally, these antagonists may also have anxiolytic (anti-anxiety) effects, making them useful in the management of anxiety disorders.
It's important to note that the specific effects and applications of 5-HT1A receptor antagonists may vary depending on the particular drug and the condition being treated. Therefore, it's crucial to consult with a healthcare professional for accurate information and appropriate use of these medications.
Drug Target R&D Trends for alverine citrate
The 5-HT1A receptor is a subtype of serotonin receptor found in the human body. It plays a crucial role in regulating various physiological and psychological processes. Activation of the 5-HT1A receptor by serotonin, a neurotransmitter, leads to inhibitory effects on neuronal activity, resulting in the modulation of mood, anxiety, and stress responses. This receptor is also involved in the regulation of sleep, appetite, and pain perception. Dysfunction of the 5-HT1A receptor has been implicated in various psychiatric disorders, including depression, anxiety disorders, and schizophrenia. Understanding the role of the 5-HT1A receptor is essential for the development of novel therapeutic interventions targeting these conditions.
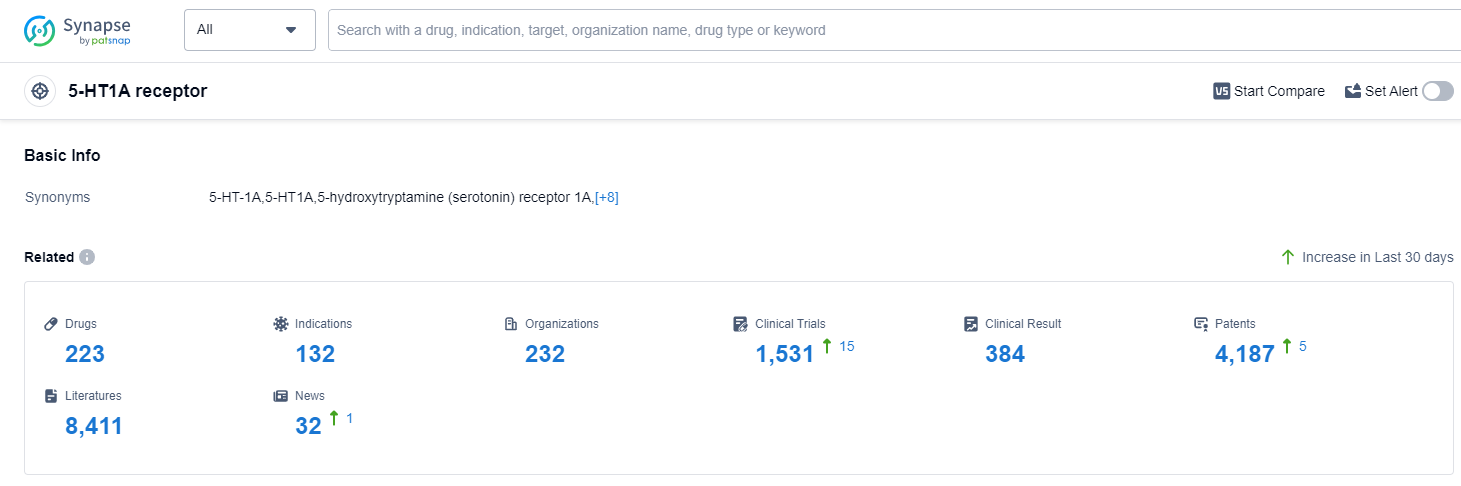
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 223 5-HT1A receptor drugs worldwide, from 232 organizations, covering 132 indications, and conducting 1531 clinical trials.
The analysis of the target 5-HT1A receptor reveals a competitive landscape with multiple companies actively developing drugs for various indications. Otsuka Holdings Co., Ltd., AbbVie, Inc., and Sumitomo Chemical Co., Ltd. are the companies with the highest number of drugs in different stages of development. The most common indication for approved drugs is Depressive Disorder, Major. Small molecule drugs are progressing rapidly, indicating intense competition in this area. The United States, China, and the European Union are the countries/locations with the highest development progress. China, in particular, has shown significant progress with several drugs in the approved stage. Overall, the target 5-HT1A receptor presents a promising area for future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Alverine Citrate is a small molecule drug developed by Dr. Reddy's Laboratories (UK) Ltd. It targets the 5-HT1A receptor and is approved for the treatment of diverticulosis, colonic, dysmenorrhea, and irritable bowel syndrome. Its first approval occurred in the United Kingdom in September 2018. This drug holds promise in the field of biomedicine and offers potential therapeutic benefits for patients with nervous system diseases and digestive system disorders.