IBI351: A Quick Look at Its R&D Progress and Clinical Results from the 2023 ESMO_ASIA
IBI351 (GFH925) is an irreversibly covalent inhibitor of KRASG12C. Previously, a pooled analysis of two phase I studies reported preliminary efficacy and safety of IBI351 (GFH925) monotherapy in metastatic colorectal cancer (CRC) harboring KRASG12C mutation (Ying Yuan, et al., ASCO 2023). On 2 Dec 2023, the updated results of these two studies with extended follow-up were presented in 2023 ESMO_ASIA.
IBI351's R&D Progress
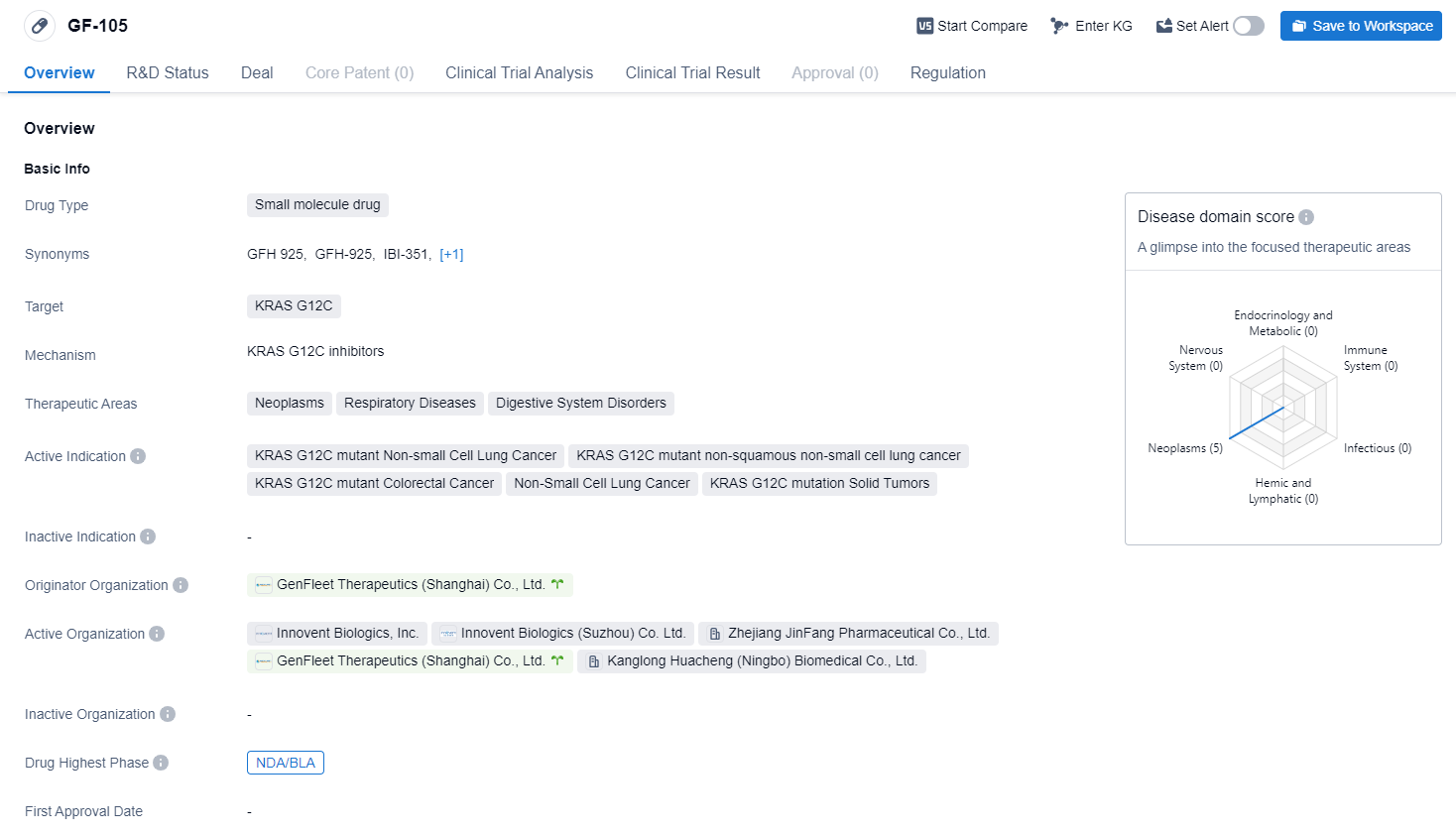
IBI351, also called GF-105, is a small molecule drug developed by GenFleet Therapeutics (Shanghai) Co., Ltd. It is designed to target the KRAS G12C mutation, which is associated with various types of cancers. The drug has shown potential therapeutic benefits in the treatment of neoplasms, respiratory diseases, and digestive system disorders.
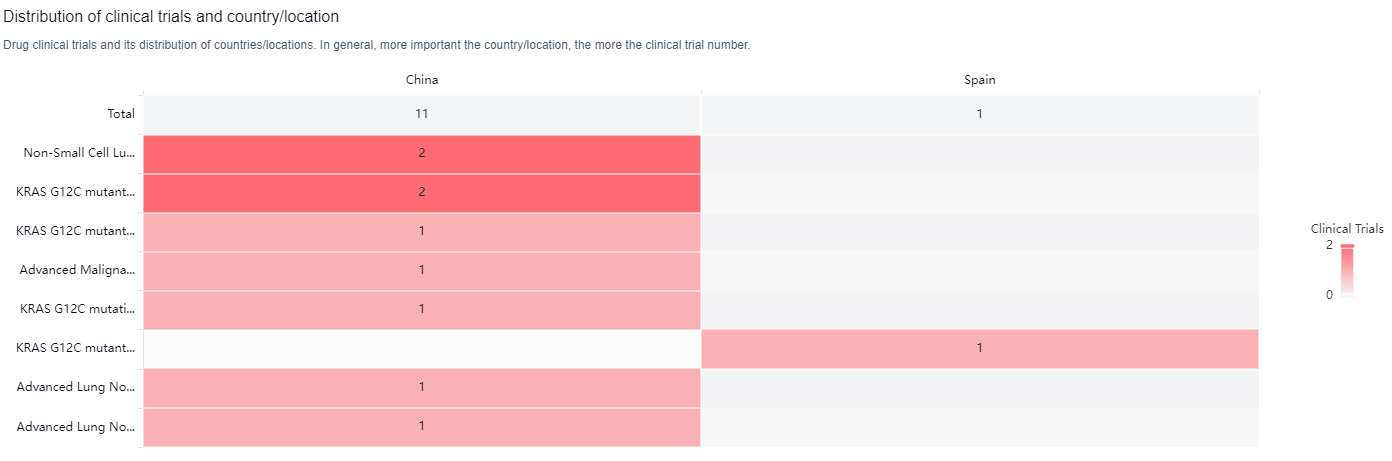
According to the Patsnap Synapse, IBI351 has reached the highest phase of development globally, with NDA/BLA status. And the clinical trial distributions for IBI351 are primarily in China and Spain. The key indication is Non-Small Cell Lung Cancer.
Detailed Clinical Result of IBI351
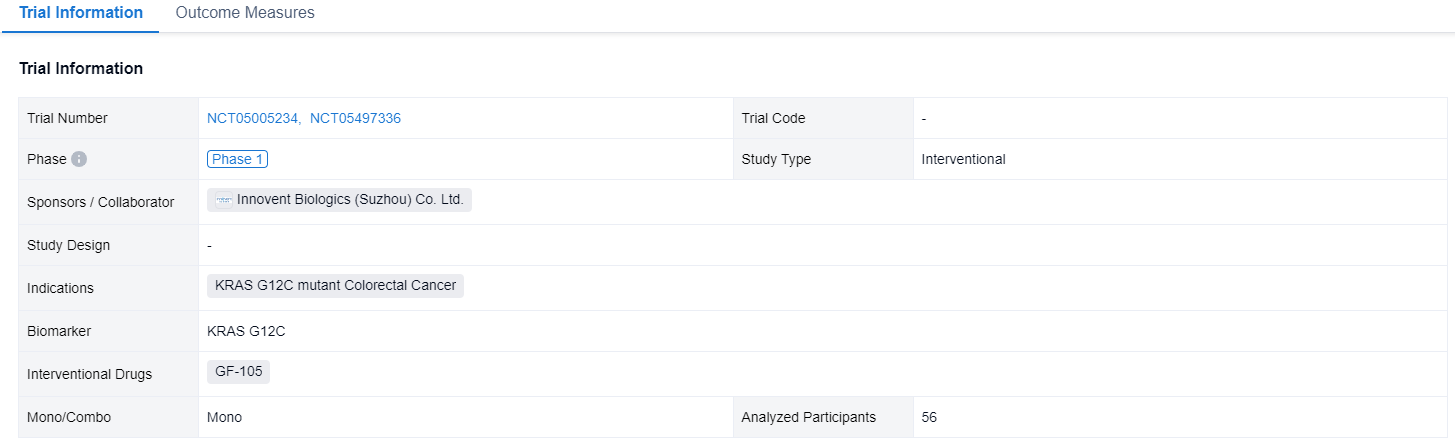
This latest clinical trial (NCT05005234; NCT05497336) was designed to investigate the efficacy and safety of IBI351 (GFH925) monotherapy in metastatic colorectal cancer harboring KRASG12C mutation.
In this study, eligible metastatic CRC patients (pts) with KRASG12C were enrolled. Pts received IBI351 (GFH925) orally at dose levels of 700mg once daily (QD) or 450/600/750mg twice daily (BID). The primary endpoint was objective response rate (ORR) assessed by investigator per RECIST v1.1.
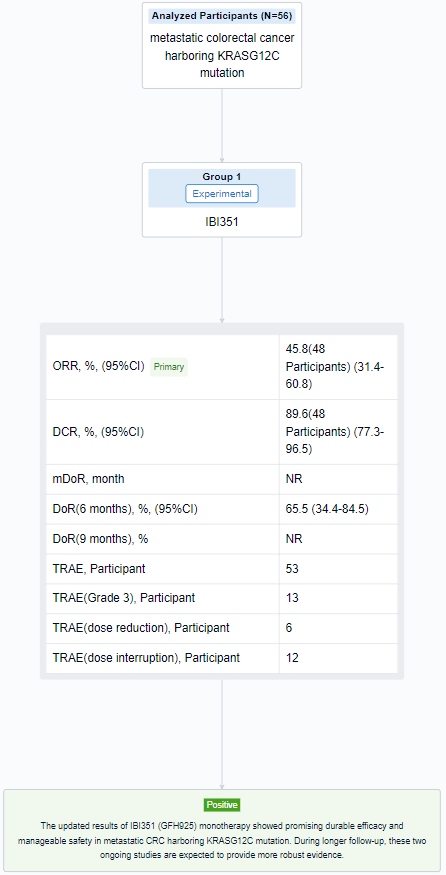
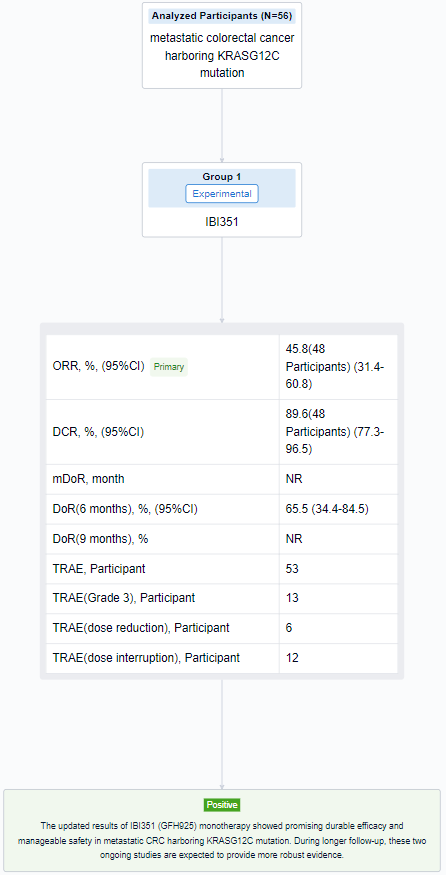
The result showed that as of June 13, 2023, a total of 56 pts were enrolled (median age: 58.0 years; male: 60.7%; ECOG PS 1: 73.2%; ≥2 prior lines of treatment: 60.7%; liver metastasis: 60.7%). The median treatment duration was 172.5 days (range: 8-481) and 26 pts (46.4%) were still on treatment. For 48 pts at 600mg BID, confirmed ORR was 45.8% (95%CI: 31.4%-60.8%) and disease control rate (DCR) was 89.6% (95%CI: 77.3%-96.5%). Median duration of response (DOR) was not reached with events occurring in 5 (22.7%) pts. DOR rates were 65.5% (95%CI: 34.4%-84.5%) at 6 months and not reached at 9 months. In pts of all dose levels, treatment-related adverse events (TRAEs) occurred in 53 (94.6%) pts while majority of them were grade 1-2. Grade 3 TRAEs occurred in 13 (23.2%) pts. Neither grade 4-5 TRAEs nor treatment-related serious adverse events (TRSAEs) occurred. The most common TRAEs included anemia (48.2%), white blood cell count decreased (30.4%), blood bilirubin increased (26.8%) and pruritus (26.8%). TRAEs leading to dose reduction and interruption occurred in 6 (10.7%) and 12 (21.4%) pts. No TRAEs leading to treatment discontinuation or death occurred.
It can be concluded that IBI351 (GFH925) monotherapy showed promising durable efficacy and manageable safety in metastatic CRC harboring KRASG12C mutation. During longer follow-up, these two ongoing studies are expected to provide more robust evidence.
How to Easily View the Clinical Results Using Synapse Database?
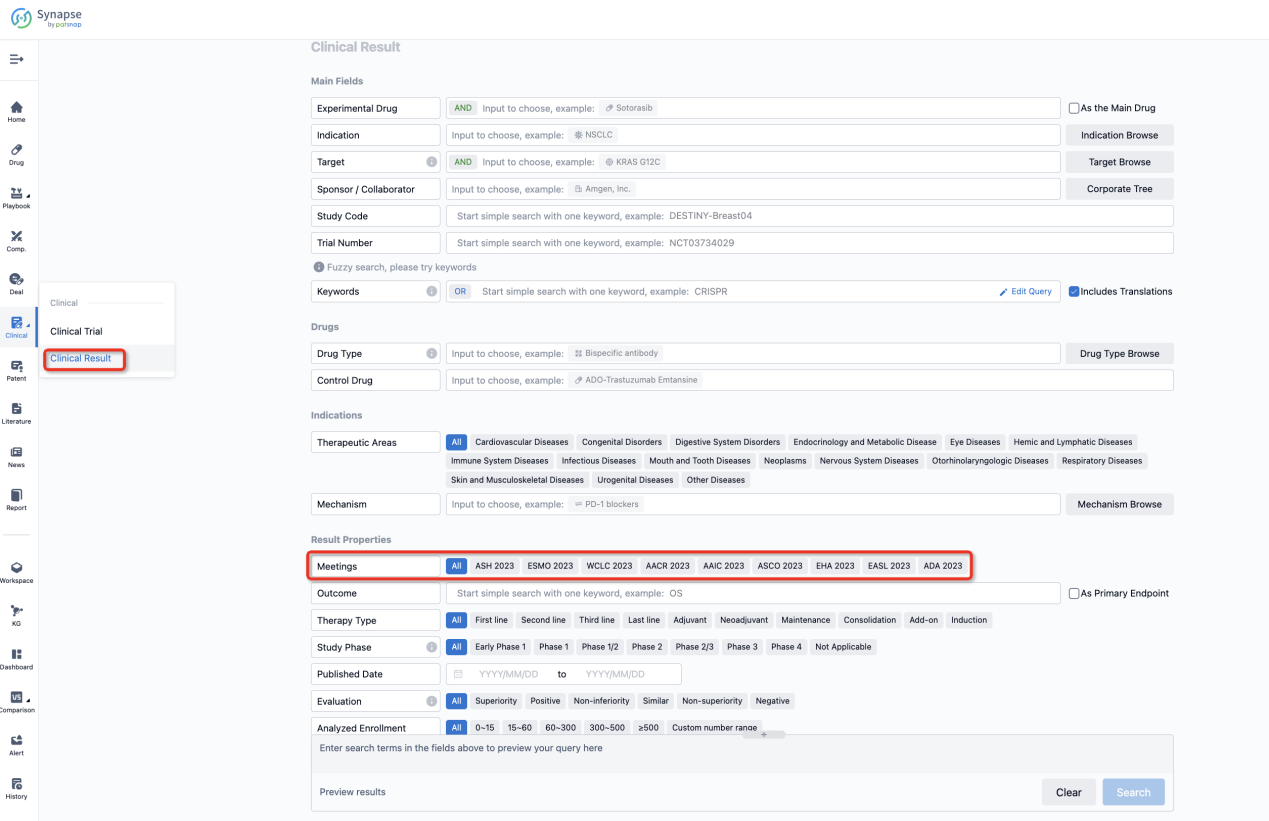
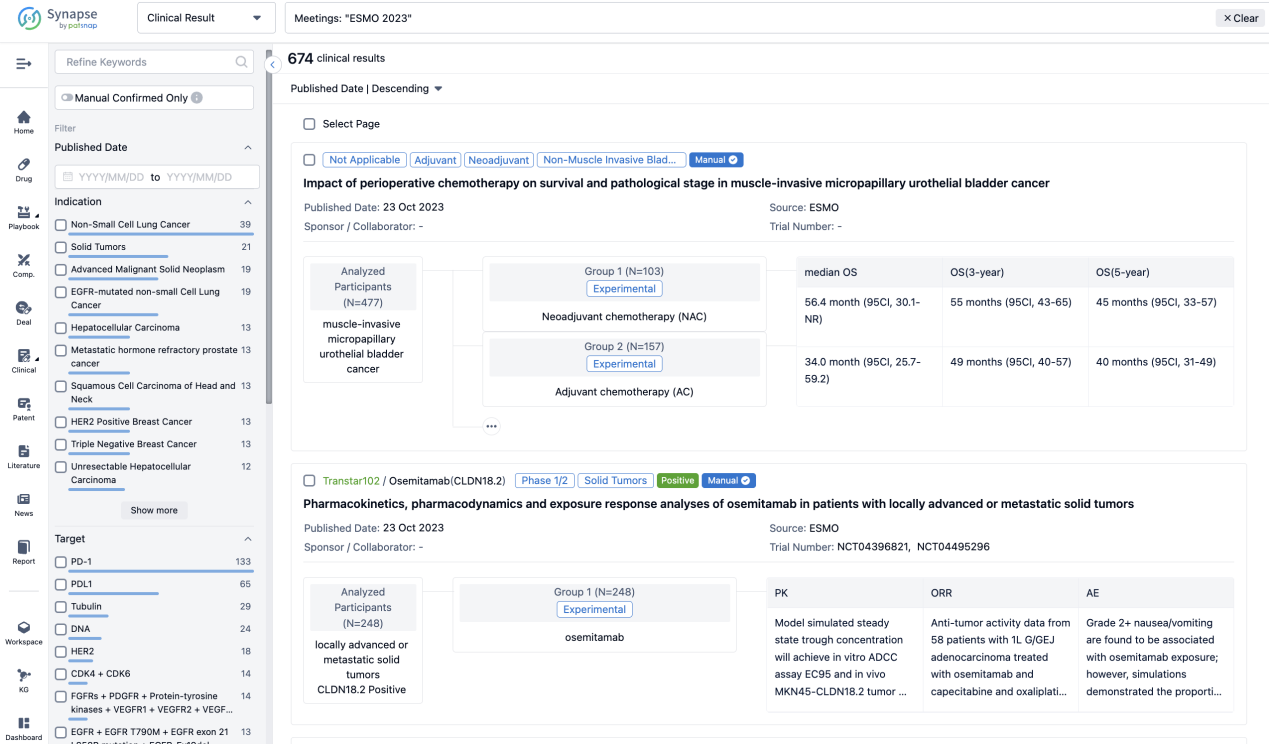
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
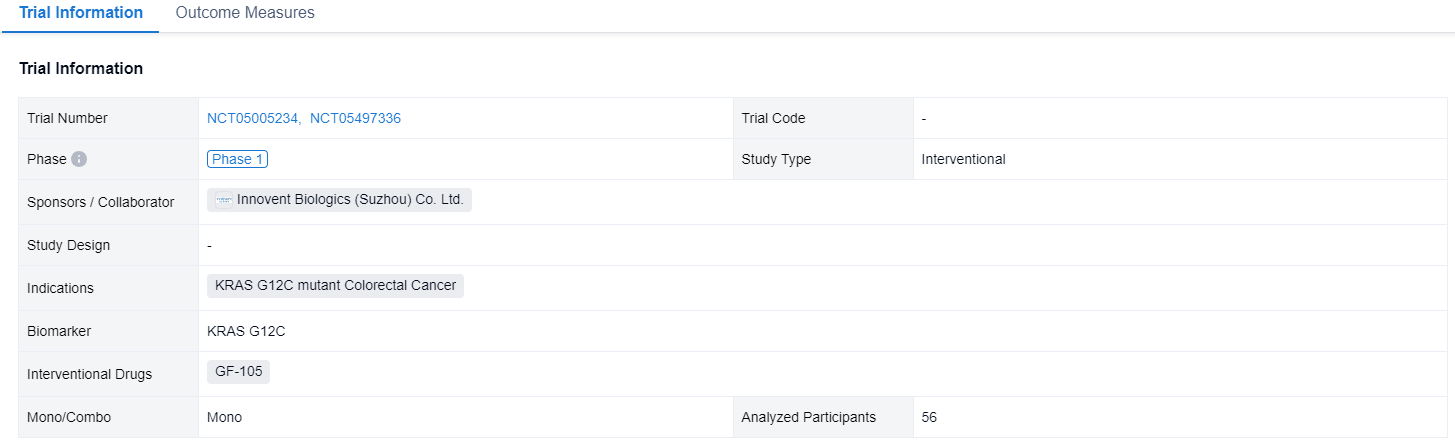
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
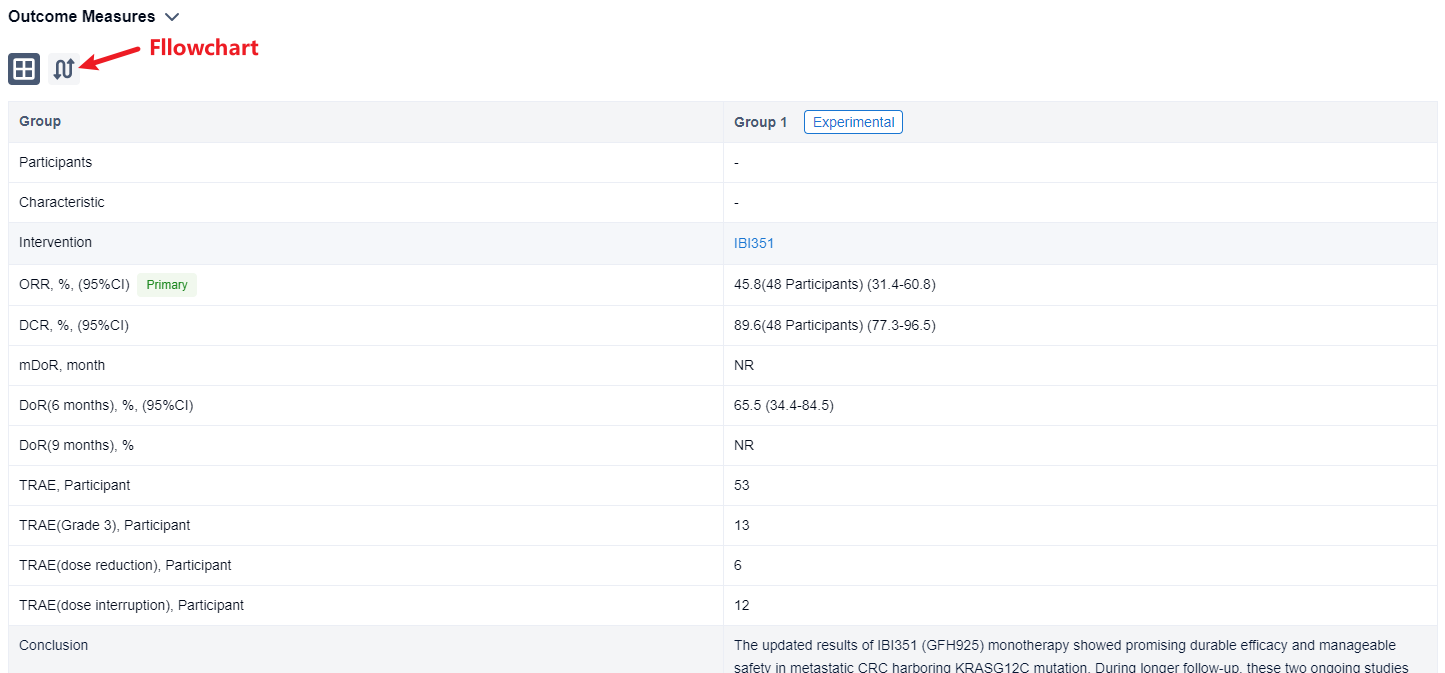
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
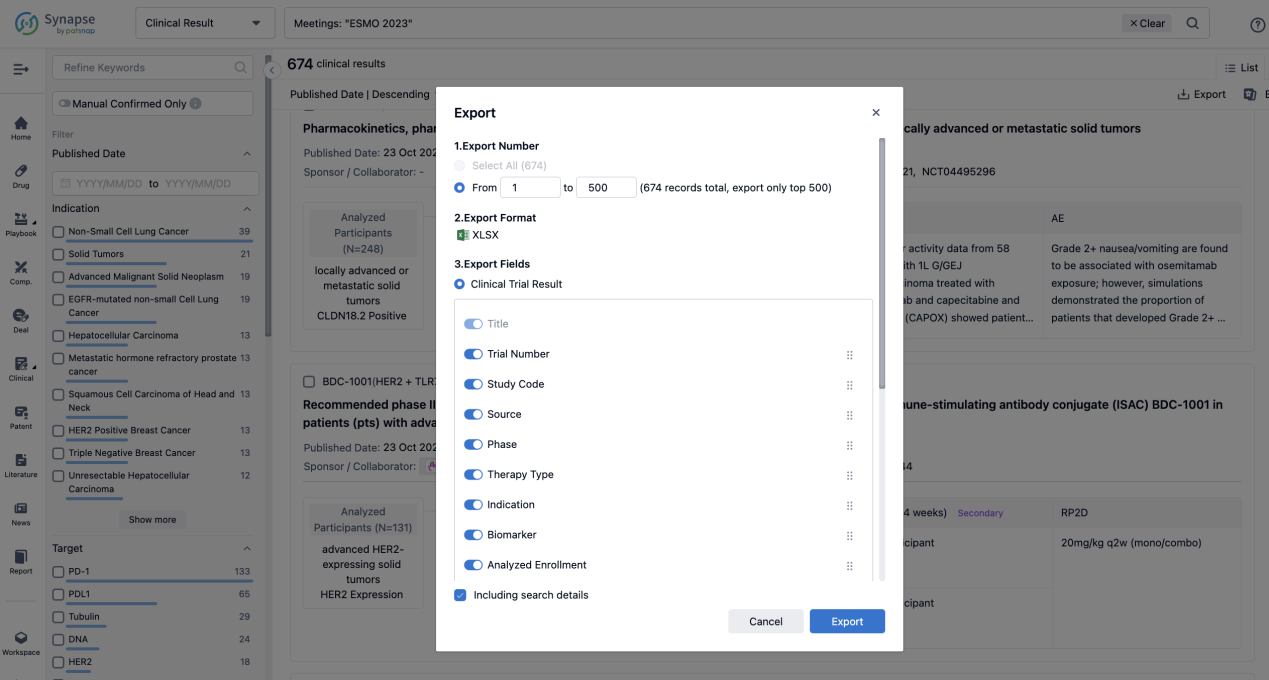
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!