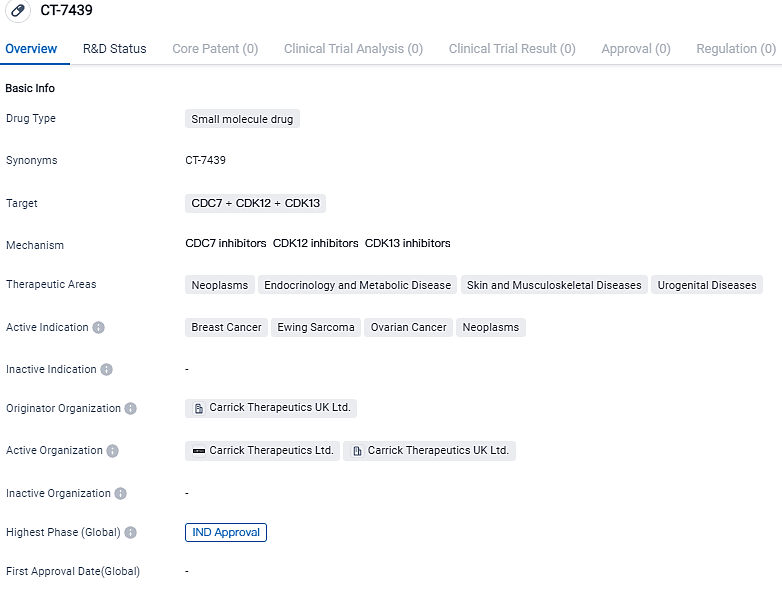
Carrick Therapeutics Declares the U.S. FDA Approval the IND for CT7439, a First-In-Class Inhibitor of CDK12/13
Carrick Therapeutics, a biopharma corporation focusing on cancer treatments, made an announcement that their IND application for CT7439, a unique cyclin dependent kinase 12/13 (CDK12/13) suppressor, has been given the green light by U.S FDA. Their ambition is to embark on a Phase 1 clinical study during the initial six months of 2024, intending to include patients diagnosed with advanced solid cancers such as those found in the breast, ovaries and Ewing's Sarcoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Receiving the FDA's approval for our IND application for CT7439, which represents Carrick's second therapeutic candidate, boosts our portfolio in oncology and will aid in assessing the potential of our encouraging preclinical outcomes in patients diagnosed with solid tumors," stated Carrick Therapeutics' CEO, Tim Pearson.
The first phase of the clinical trial is based on a modular structure, commencing with a dosage elevation in the initial administration of CT7439 to patients. The primary clinical assessment will be centered on safety and pharmacokinetics, accompanied by a chance for early evidence of efficacy through a blood-based pharmacodynamic assay of the homologous recombination repair (HRR) pathway.
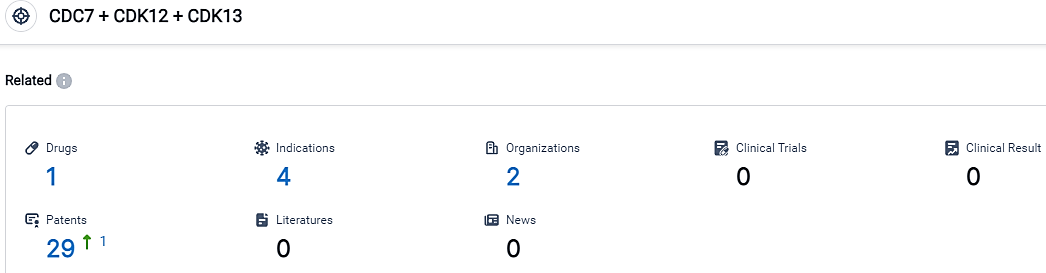
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 13, 2023, there are 1 investigational drugs for the CDC7 and CDK12 and CDK13 target, including 4 applicable indications,2 R&D institutions involved, and as many as 29 patents.
CT7439 is an inhibitor of CDK12/13 as well as a 'glue degrader' of Cyclin-K, which is the obligate co-factor for CDK12/13, giving both first-in-class and best-in-class potential. The twin modality significantly boosts the competence of the compound, resulting in the obstruction of DNA repair at the level of transcription. CDK12/13 control gene transcription by stimulating RNA Polymerase II. It carries potential to work in sync with other DDR-targeting agents like the PARP inhibitors across numerous forms of cancer like breast, ovarian and Ewing’s Sarcoma.