Janssen presents an application for Marketing Authorisation to the EMA, requesting endorsement for Erdafitinib's utilization in the management of patients
The Janssen Pharmaceutical Companies declared their filing of a Marketing Authorisation Application with EMA in the hopes of acquiring greenlight for erdafitinib. This is aimed at the treatment of adult patients who have undergone disease progression during or after at least one line of therapy containing a PD-1 or PD-L1 inhibitor; they are dealing with locally advanced unresectable or metastatic urothelial carcinoma, which holds susceptible fibroblast growth factor receptor 3 (FGFR3) genetic mutations.
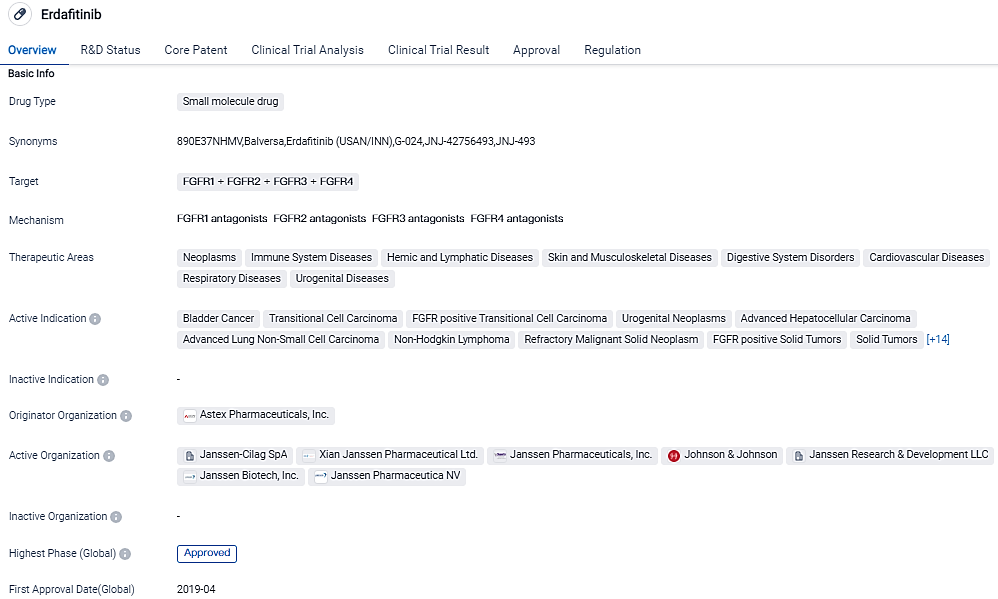
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Europe's bladder cancer is among the highest in the world, with over 203,000 patients receiving a diagnosis in 2020. UC is the primary variant, and as many as 20% of those with metastatic UC have FGFR alterations. Patients suffering from mUC, which includes FGFR-driven tumors, are met with an especially bleak outlook, and innovative treatments are greatly needed. Just 8% of patients diagnosed in the later, metastatic stages, will survive past five years.
" patients with advanced UC, including FGFR-driven tumors, continue to face grim prognoses, and their treatment options are limited. Thus, the search for novel, targeted treatments is crucial", said Martin Vogel, EMEA Therapeutic Area Lead Oncology, Janssen-Cilag GmbH. "We are encouraged by the possibility of delivering groundbreaking, individualized treatments to market as part of our broader aim of making this complicated disease more manageable and, eventually, treatable."
"This application process, coupled with Janssen’s ongoing study of erdafitinib, emphasizes our dedication to delivering crucial targeted treatments in areas of extreme need, including terminal diseases like metastatic UC", remarked Kiran Patel, M.D., Vice President, Clinical Development, Solid Tumors, Janssen Research & Development, LLC.
In April 2019, erdafitinib received preliminary approval from the U.S. FDA for treating adult patients with metastatic or mUC and FGFR3 or FGFR2 genetic alterations who have worsened during or after at least one previous platinum-containing chemotherapy. On August 29, Janssen applied for a sNDA with the U.S. FDA striving for full approval of erdafitinib based on Cohort 1 of the Phase 3 THOR trial.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
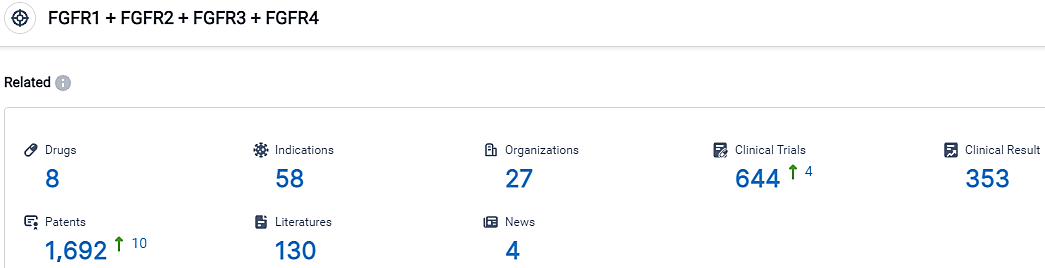
According to the data provided by the Synapse Database, As of September 13, 2023, there are 8 investigational drugs for the FGFR1 and FGFR2 and FGFR3 and FGFR4 target, including 58 applicable indications,27 R&D institutions involved, with related clinical trials reaching 644,and as many as 1692 patents.
Urothelial carcinoma, also known as transitional cell carcinoma, starts in the innermost lining of the bladder. Almost all bladder cancers – more than 90 percent – are UCs. AstraZeneca PLC, Bristol Myers Squibb Co., Johnson & Johnson, Roche Holding AG are among the companies with the highest stage of development in this indication. The targets of drugs for Urothelial carcinoma include IFNAR, Top II, Tubulin, PD-1, FGFR1 + FGFR2 + FGFR3 + FGFR4 and EGFR. The indication Urothelial carcinoma has a competitive landscape with diverse R&D activities and potential for future development.