Opus Genetics reports the administration of their gene therapy OPGx-LCA5 to the first participant of their phase 1/2 clinical trial
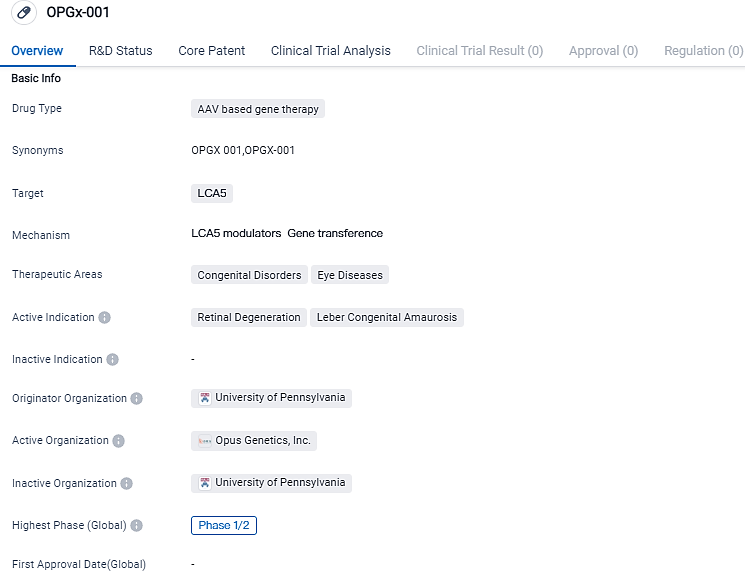
Opus Genetics, a company focused on developing gene therapies for inherited eye diseases, has administrated to the first patient in its first-in-human Phase 1/2 clinical trial for OPGx-LCA5. This innovative therapy, which uses adeno-associated virus 8 make-up, aims to transport a functioning LCA5 gene to the external retina. The treatment is intended for sufferers of Leber congenital amaurosis caused by biallelic mutations in the LCA5 gene (LCA5).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
LCA5 is a early-onset retinal decay impacting around one in 1.7 million individuals in the U.S., with no officially approved therapies available at the moment for those with vision loss related to LCA5.
"Treating our first patient confirms Opus as a firm entering the clinical-stage, and showcases our continuous pursuit to introduce groundbreaking gene therapies for inherited retinal disorders," stated Opus's CEO, Ben Yerxa, Ph.D. "We look forward to advancing the testing of this potentially revolutionary treatment for patients affected by LCA5."
The ongoing Phase 1/2 trial, which is an open-label and dose- escalation study, is assessing the subretinal administration of OPGx-LCA5 on nine adults with LCA5. The trial aims to study the safety and preliminary effectiveness of OPGx-LCA5 on patients suffering from inherited retinal decay as a result of biallelic mutations in the LCA5 gene. After the FDA has validated adult safety, Opus plans to include a pediatric group in the study.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
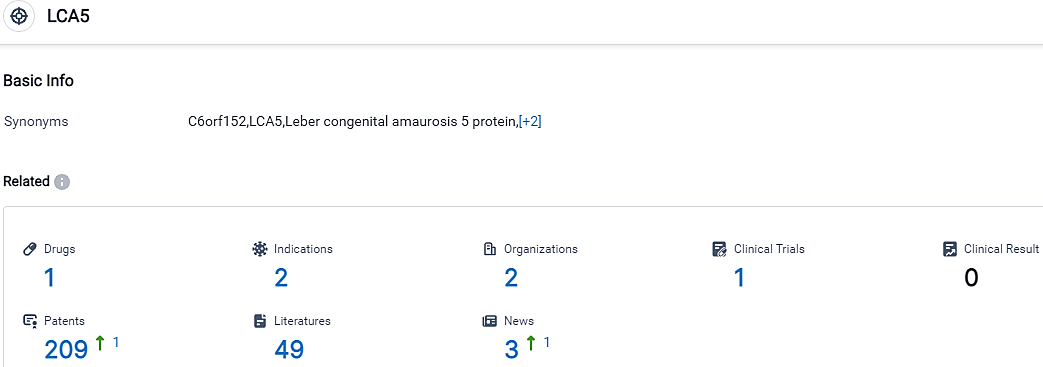
According to the data provided by the Synapse Database, As of September 13, 2023, there are 1 investigational drugs for the LCA5 target, including 2 applicable indications, 2 R&D institutions involved, with related clinical trials reaching 1,and as many as 209 patents.
For indication of retinal degeneration, Novartis AG and Roche Holding AG are the leading companies with the highest number of drugs in various development phases. VEGF-A is the most targeted pathway, indicating its significance in the treatment of retinal degeneration. This analysis provides valuable insights into the current competitive landscape and future development of the indication Retinal Degeneration.