CDR-Life Begins Phase 1 Trial of CDR404 for Solid Tumors at ASCO
CDR-Life Inc. has started enrolling participants for the Phase 1 clinical trial of CDR404, their leading candidate being developed as a targeted immunotherapy for the treatment of solid tumors. An abstract about CDR404 has been approved for online presentation at the 2024 American Society of Clinical Oncology Annual Meeting, scheduled to take place from June 3 to June 4 in Chicago, Illinois.
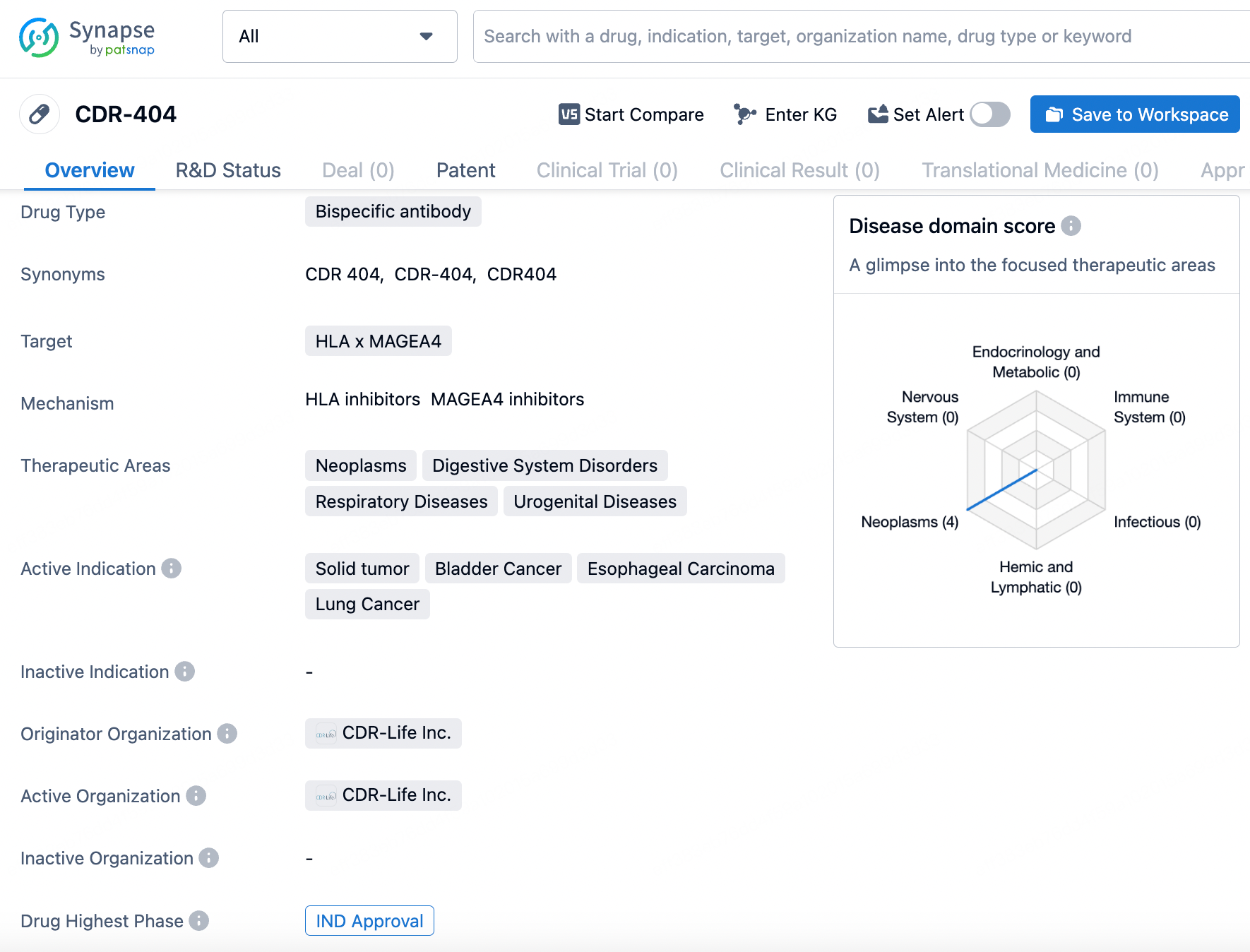
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Leveraging its proprietary M-gager® technology, CDR404 stands out as an innovative, antibody-based bivalent and bispecific MAGE-A4 T-cell engager designed to treat MAGE-A4 positive solid tumors.
"We are delighted with the advancements achieved following the approval of our Investigational New Drug application by the U.S. FDA earlier this year, along with the subsequent authorization of the Clinical Trial Application in Denmark. We look forward to sustaining this positive momentum," remarked Christian Leisner, Ph.D., Chief Executive Officer at CDR-Life. "This significant milestone brings us closer to our objective of providing a superior off-the-shelf therapy for cancer patients in critical need."
The ASCO abstract outlines findings from a study evaluating the biomarkers that predict the efficacy of CDR404 against non-small cell lung cancer tumors.
CDR-404 precisely targets HLA and MAGEA4 and is aimed at treating various conditions, including neoplasms, digestive system disorders, respiratory diseases, and urogenital diseases. The targeted indications for CDR-404 encompass advanced malignant solid neoplasm, bladder cancer, esophageal carcinoma, and lung cancer. According to the most recent data, CDR-404 is currently in Phase 1 of clinical trials.
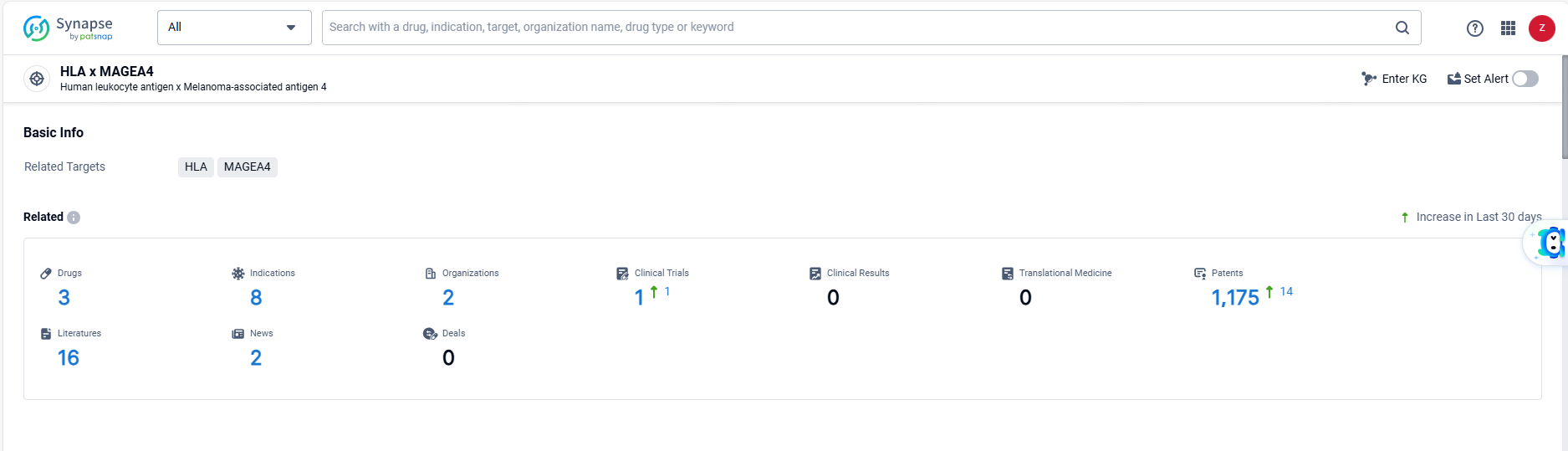
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 3, 2024, there are 3 investigational drugs for the HLA and MAGEA4 targets, including 8 indications, 2 R&D institutions involved, with related clinical trials reaching 1, and as many as 1175 patents.
CDR-404 represents a significant advancement in the field of biomedicine, particularly in the area of cancer treatment. The drug's bispecific antibody design, coupled with its targeting of multiple therapeutic areas and active indications, positions it as a potential candidate for addressing unmet medical needs in the treatment of various cancers and related disorders. Further clinical development and evaluation will be necessary to determine the full potential of CDR-404 in improving patient outcomes in these disease areas.