Chemical Modifications and Delivery Strategies of ASOs and siRNAs in Gene Therapy
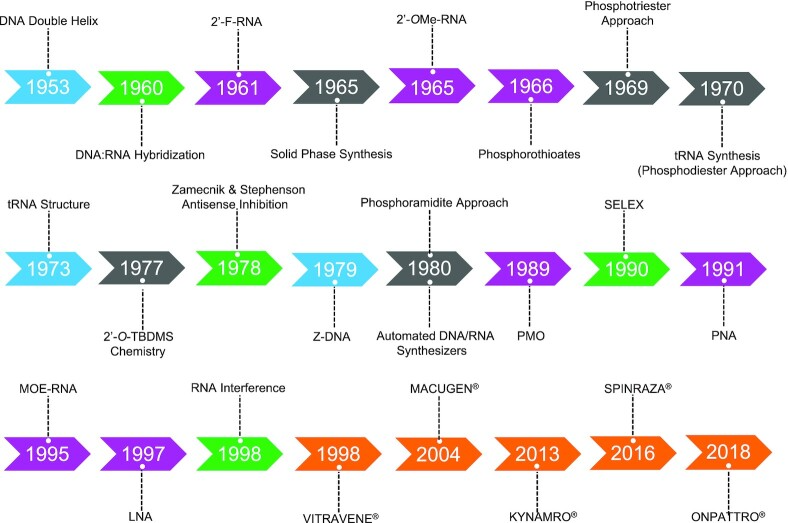
Antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), as vital components of precision medicine, have demonstrated tremendous potential in the field of gene therapy. To enhance the efficacy and safety of these therapies, researchers have developed various chemical modifications and technologies, such as phosphorothioate (PS) modifications, 2'-O-methyl modifications, 2'-fluoro modifications, and locked nucleic acid (LNA) modifications. These modifications not only improve the stability of the therapeutics but also enhance their binding affinity to target RNA. Additionally, innovative delivery strategies, including lipid nanoparticles (LNPs), cationic liposomes, and polymer nanoparticles, further address challenges encountered during in vivo delivery, such as susceptibility to nuclease degradation and difficulty in cellular uptake.
By leveraging Patsnap Bio, homology alignment can be performed to evaluate the specificity of designed oligonucleotides, thereby preventing off-target effects. Furthermore, the published literature and patent information contained in the database enable researchers to stay updated on the latest technological developments, inspiring new research ideas. When developing ASO or siRNA therapies for specific diseases, developers can reference research findings on relevant disease models within the database to optimize drug design strategies.
Shared Chemical Modifications of ASOs and siRNAs
1.PS Modifications
PS modification involves replacing one of the oxygen atoms in the nucleotide backbone with a sulfur atom, thereby increasing the drug's stability and cellular penetration capability. This modification makes the therapeutic less susceptible to nuclease degradation and enhances cellular uptake.
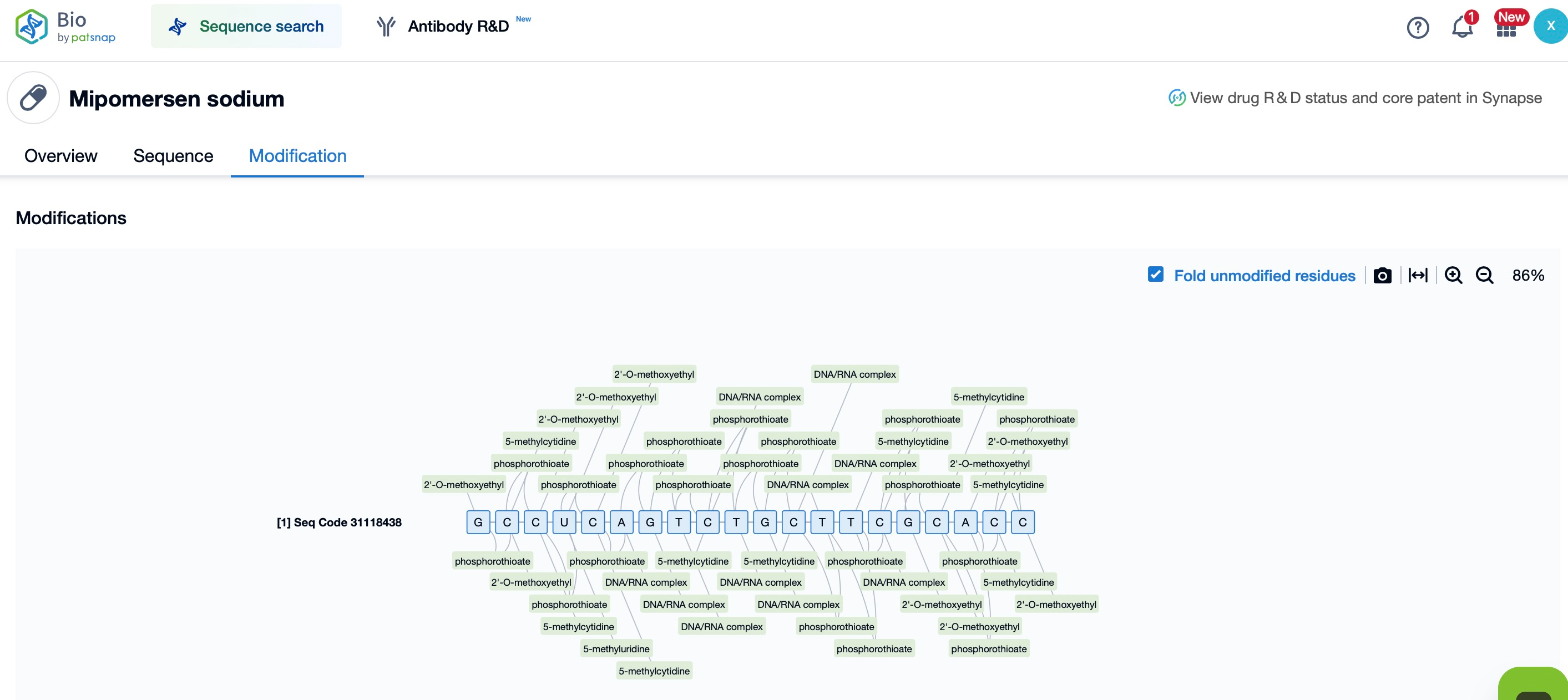
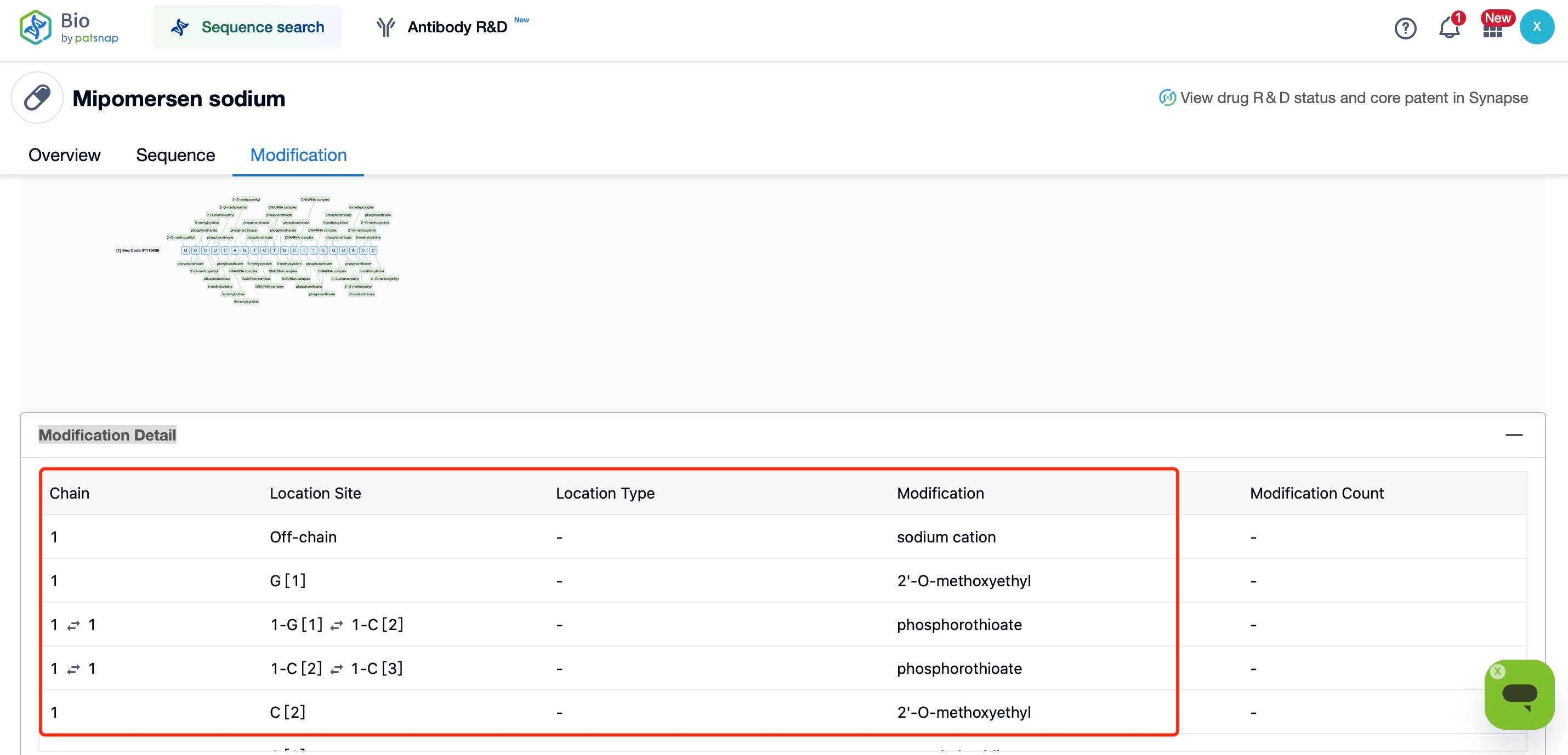
For example, Mipomersen (Kynamro), an ASO drug used to treat hypercholesterolemia, incorporates PS modifications. These modifications improve its stability in the bloodstream and enable effective binding to target mRNA, reducing the levels of apoB-100, the precursor of low-density lipoprotein (LDL) receptors. As a result, Mipomersen effectively lowers LDL cholesterol levels in the blood. Through PS modification, Mipomersen exhibits increased resistance to nuclease degradation in vivo, thereby achieving its therapeutic effect.
2. 2'-O-Methyl Modification
The 2'-O-Methyl modification introduces a methyl group at the 2' position of the nucleotide, which enhances the drug's thermal stability, binding affinity to RNA, and resistance to nuclease degradation. This modification stabilizes the therapeutic molecule inside cells, protecting it from enzymatic breakdown and improving its ability to efficiently bind to target RNA molecules, thereby achieving a more effective gene-silencing effect.
For example, Inotersen (brand name Tegsedi) is an ASO drug used to treat hereditary transthyretin-mediated amyloidosis (hATTR). hATTR is a rare disorder caused by the deposition of unstable transthyretin (TTR) protein in tissues, leading to peripheral neuropathy and cardiac complications. Inotersen utilizes the 2'-O-Methyl modification to enhance its in vivo stability, ensuring efficient targeting and degradation of TTR mRNA, which reduces the production of pathogenic TTR protein. This modification allows Inotersen to resist intracellular nuclease degradation, enabling longer intracellular persistence and sustained inhibition of TTR gene expression. Additionally, the 2'-O-Methyl modification increases binding affinity to target mRNA, allowing therapeutic effects at lower concentrations. Collectively, these properties make Inotersen an effective treatment option, offering hope for patients with hATTR.
3. 2'-Fluoro Modification
The 2'-Fluoro modification involves introducing a fluorine atom at the 2' position of the nucleotide, enhancing the drug's thermal stability, RNA-binding ability, and resistance to degradation. This chemical modification not only improves oligonucleotide stability but also alters its physicochemical properties, facilitating cellular uptake.
For instance, Givosiran (brand name Givlaari) is an RNA interference (RNAi) drug used to treat acute hepatic porphyria (AHP). Its siRNA molecule incorporates multiple chemical modifications, including 2'-Fluoro. The addition of 2'-Fluoro enhances the stability of the siRNA, prolonging its survival in vivo and improving overall drug efficacy. This modification ensures that Givosiran can effectively reach target tissues while maintaining sufficient activity to execute its gene-silencing function. Beyond improving drug stability, the 2'-Fluoro modification positively influences binding affinity to the target RNA. By integrating such chemical adjustments, scientists can design more efficient and durable therapeutic candidates to address chronic diseases requiring long-term or intermittent treatment. Even in drugs like Givosiran, which already possess optimized delivery mechanisms, the 2'-Fluoro modification remains a critical component for enhancing therapeutic outcomes.
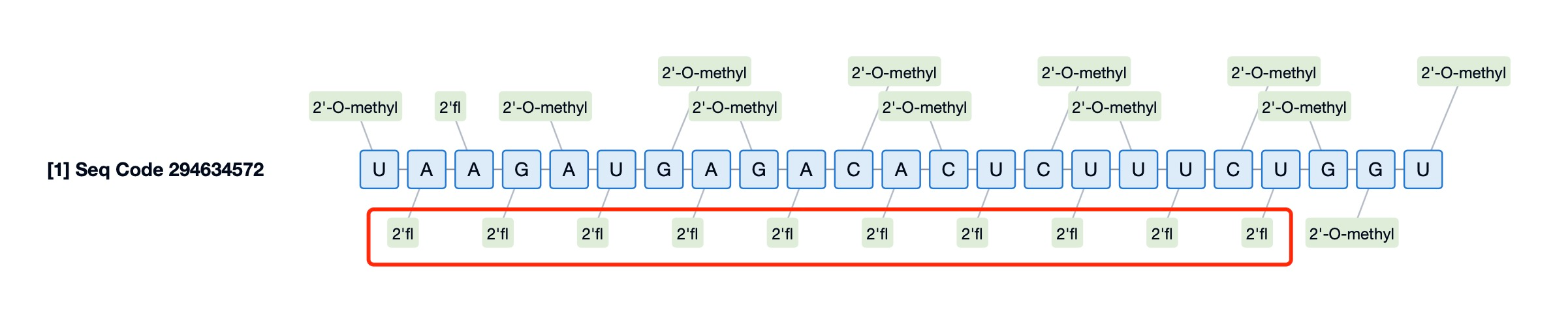
4. Locked Nucleic Acid (LNA) Modification
The LNA modification introduces an additional bridging bond into the ribose ring, forming a stable five-membered ring. This structural enhancement increases the drug's thermal stability and its binding affinity to RNA. While LNA modification is widely applied in siRNAs, it is equally significant in ASOs. For example, certain ASO drugs incorporate LNA modifications to improve target RNA affinity, ensuring effective inhibition of target gene expression.
5. Gapmer Design
Gapmer design is a specialized oligonucleotide structure primarily used in ASOs. The design involves combining nucleotide fragments with different chemical modifications to optimize therapeutic effects. Typically, the central segment consists of unmodified standard DNA nucleotides, which are responsible for specific pairing with the target RNA sequence. This segment determines the oligonucleotide's specificity, enabling precise recognition and binding to the target RNA. Chemically modified nucleotides, such as PS or LNA, are used at both ends of the structure to enhance RNA binding affinity, increase stability, and improve resistance to nuclease degradation.
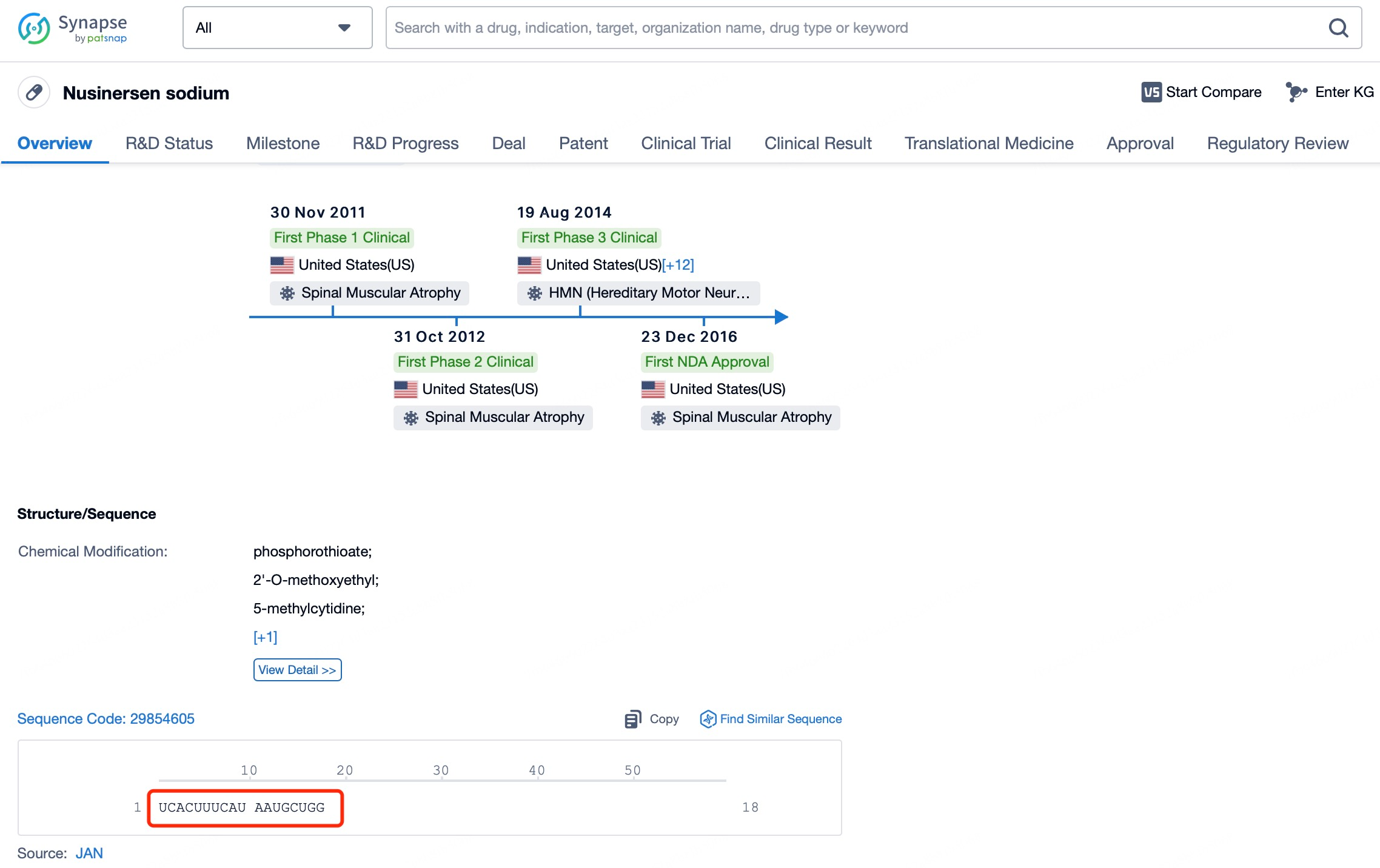
For instance, Nusinersen (brand name Spinraza) is an ASO drug used to treat spinal muscular atrophy (SMA). This drug adopts a Gapmer design, with a central segment of unmodified DNA nucleotides for specific pairing with the target RNA sequence, and chemically modified nucleotides such as PS or LNA at both ends. These modifications enhance stability and binding to the target RNA, ensuring effective modulation of SMN2 gene pre-mRNA splicing to increase the production of functional SMN protein.
Delivery Methods for ASOs and siRNAs
To improve the bioavailability and stability of ASOs, researchers have developed various delivery techniques and chemical modification strategies. Modifications such as phosphorothioate (PS) or LNA not only enhance the stability of ASOs, preventing their degradation by nucleases, but also improve their binding affinity to target RNA. These chemical modifications confer favorable pharmacokinetic properties, allowing ASOs to cross cell membranes directly without requiring additional delivery vehicles, simplifying the delivery process and reducing potential side effects. For example, PS modification is a common method that significantly increases the stability and bioavailability of ASOs, enabling them to exert therapeutic effects in multiple tissues and organs.
To further enhance the efficacy and targeting capabilities of ASOs, researchers have employed carrier technologies such as liposomes or nanoparticles. These carriers protect ASOs from nuclease degradation and facilitate their transport across cellular barriers to target locations. By leveraging these combined strategies, ASOs can effectively reach target cells in vivo, exert therapeutic effects, and minimize off-target effects and toxicity. For instance, ASO drugs like Nusinersen (brand name Spinraza), which utilize advanced delivery systems and chemical modifications, have achieved remarkable clinical success, showcasing the immense potential of combining chemical modifications with modern delivery technologies.
One of the primary challenges for the clinical application of siRNAs is the effective delivery to target tissues. To address this, researchers have developed multiple delivery strategies, including encapsulating siRNAs in lipid nanoparticles (LNPs) to ensure stability in the bloodstream and promote cellular uptake. Additionally, cationic liposomes, polymer nanoparticles, and both viral and non-viral vectors are being explored as delivery systems. These advancements help overcome the challenges associated with in vivo siRNA applications, driving progress in RNA interference (RNAi) therapies.
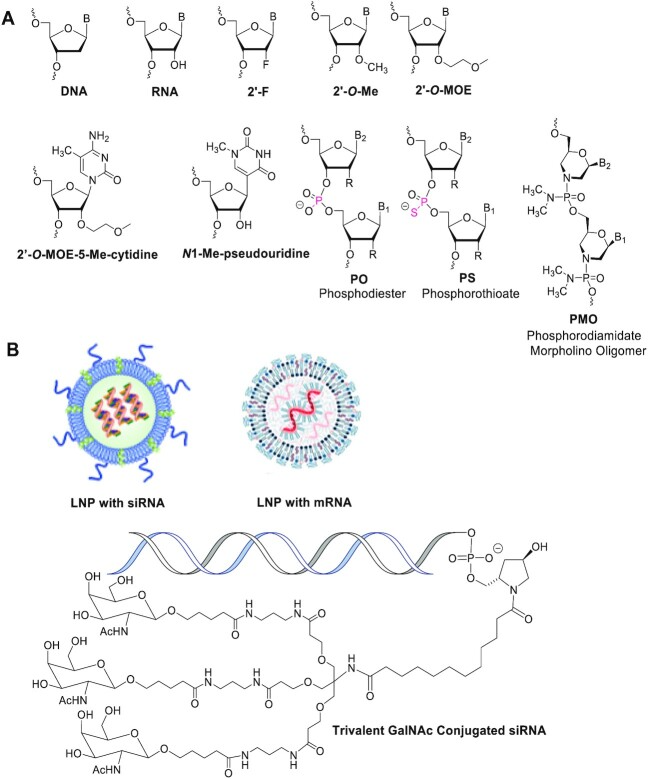
The nanoscale structures formed by LNPs protect siRNA from degradation by intracellular nucleases, ensuring stability during the delivery process. The surface components of LNPs interact with receptors on the cell surface, triggering endocytosis and facilitating the successful entry of siRNA into cells. By optimizing the composition and structure of LNPs, the uptake rate of siRNA in target cells can be significantly improved, enhancing its gene-silencing efficacy. Through surface modifications and advanced techniques, LNPs can achieve targeted delivery to specific tissues or cell types, minimizing off-target effects and associated side effects. These characteristics make LNPs an ideal choice for siRNA delivery, particularly in treatments requiring highly specific gene silencing. As delivery technologies continue to advance, LNPs and similar carriers will further drive the clinical application of siRNA therapeutics, offering innovative solutions for treating various diseases.
For siRNA delivery, liposomes or nanoparticle carriers are often required to improve cellular uptake efficiency. These carriers not only protect siRNA from nuclease degradation but also enable its efficient passage across the cell membrane into the cytoplasm, where gene silencing occurs. Specifically, LNPs encapsulate siRNA and facilitate its internalization by interacting with cell surface receptors, thereby improving intracellular delivery efficiency. This ensures that siRNA reaches its appropriate intracellular location to perform its function. These advanced delivery technologies pave the way for broader applications of siRNA therapies and suggest significant breakthroughs in personalized medicine.
Summary
ASOs and siRNAs serve as critical tools in modern gene therapy and play a pivotal role in precision medicine. By targeting specific RNA sequences to regulate gene expression, they open new avenues for treating a range of genetic diseases. To overcome challenges such as low stability, poor cellular uptake, and off-target effects, scientists have developed innovative chemical modifications and delivery technologies. Modifications like phosphorothioate (PS), 2'-O-methyl, 2'-fluoro, and LNA not only enhance the stability of oligonucleotide-based drugs but also strengthen their binding affinity to target RNA, improving therapeutic outcomes. Additionally, delivery systems such as LNPs, cationic liposomes, and polymeric nanoparticles effectively protect ASOs and siRNAs from nuclease degradation while facilitating their intracellular delivery to ensure they reach the intended site of action.
Patsnap Bio plays an indispensable role in the research and development of ASOs and siRNAs. It provides extensive biological sequence information, supporting researchers in designing and optimizing genes and oligonucleotides. Moreover, it hosts a vast collection of literature and patent data, offering invaluable knowledge resources to R&D professionals. Through Patsnap Bio, researchers can gain deep insights into existing research outcomes, evaluate the effectiveness of different chemical modifications, optimize delivery vehicle designs, and explore new therapeutic strategies. These comprehensive research tools and services significantly accelerate the translation of basic research into clinical applications, laying a solid foundation for advancing gene therapy technologies.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Refrence
Egli, M. & Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res 51, 2529-2573 (2023). https://doi.org:10.1093/nar/gkad067