Chipscreen NewWay Begins Phase 1 Trial of NWY001 for Cancer Immunotherapy
On January 5, 2024, Chengdu Chipscreen NewWay Biosciences Co., Ltd. revealed that Sun Yat-Sen University Cancer Center in China, the institution spearheading the trial, commenced the first patient treatment in a stage I clinical study with the investigational PD-1/CD40 bispecific antibody dubbed NWY001.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This study is a multi-site, non-blinded, non-randomized, multiple-dosage, initial phase clinical assessment aimed at determining the safety profile, acceptable dose range, initial responses, pharmacokinetic characteristics, and the identification of potential biological markers linked to the treatment with NWY001 in individuals carrying progressive solid malignancies.
NWY001 represents the pioneering bispecific antibody targeting PD-1/CD40 to be under clinical analysis. Functionally, this bsAb engages both PD-1 and CD40, with the potential to stimulate the CD40 pathway in response to PD-1 engagement, potentially diminishing the adverse effects seen with CD40-activating monoclonal antibodies. The anticipation is that this therapeutic agent can shift immunologically "cold" tumors into more responsive "hot" tumors, thereby heightening the response of cancer patients to PD-(L)1 blocking antibodies, encompassing those who have resistance to PD-(L)1 blockade.
Dr. Bin Liu, the Chief Scientific Officer at Chipscreen NewWay, remarked on the occasion of the first patient receiving treatment, noting it as a pivotal moment in the research journey of NWY001. He highlighted the agent's innovative mode of action as a means to possibly bypass the resistance or adverse reactions commonly associated with singular PD-(L)1 blockades, CD40 stimulation, or the combination therapeutics, potentially offering a therapeutic advantage to a broader patient populace.
On the 27th of February in the year 2023, Biocytogen Pharmaceuticals Co., Ltd. publicized that its fully owned subsidiary, Eucure Biopharma Co., Ltd., had secured an exclusive right to license with Chipscreen NewWay Biosciences, a dependent subsidiary of Shenzhen Chipscreen Biosciences Co., Ltd. This agreement is for the further clinical progression and subsequent commercial dissemination of the bispecific antibody YH008 (NWY001) across Greater China.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
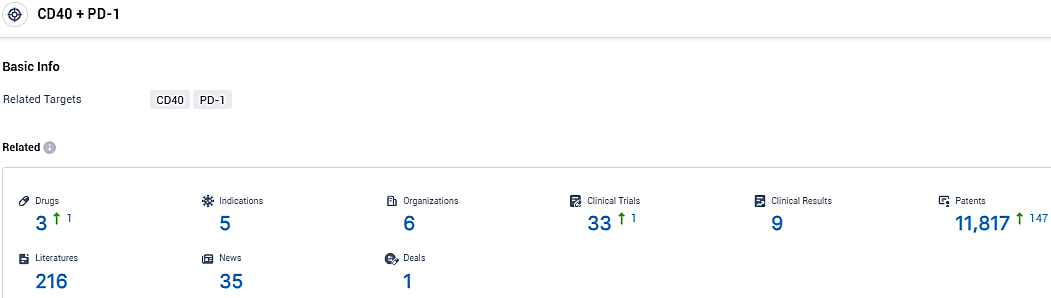
According to the data provided by the Synapse Database, As of January 19, 2024, there are 3 investigational drugs for the PD-1/CD40 target, including 5 indications, 6 R&D institutions involved, with related clinical trials reaching 33, and as many as 11817 patents.
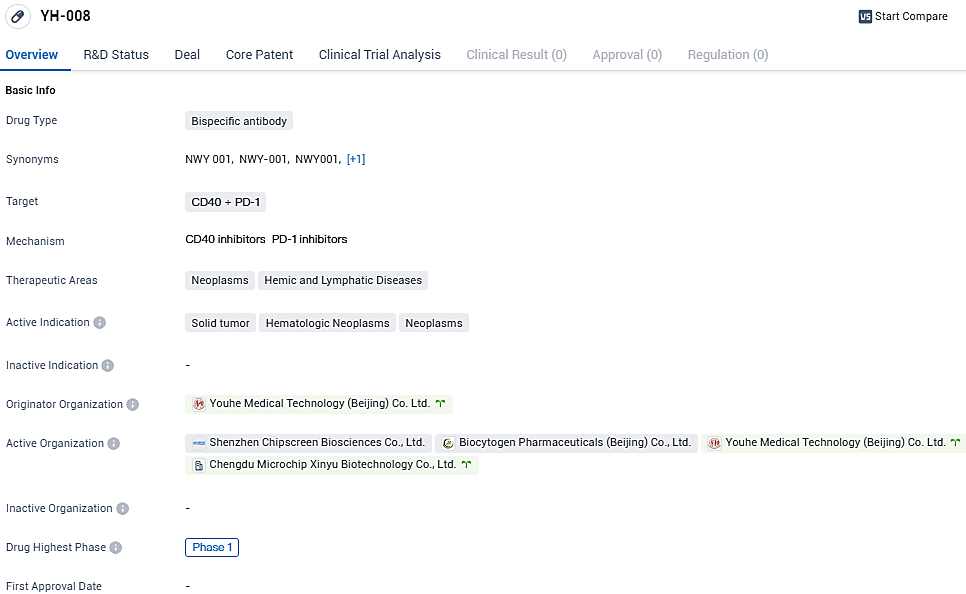
YH-008 targets CD40 and PD-1 and is being developed for the treatment of neoplasms, hemic, and lymphatic diseases. The drug is currently in Phase 1 of clinical development, indicating its early stage of testing in humans. Further studies are needed to evaluate its efficacy and safety profile.