Exploring the Latest ADC Deal by Ambrx Biopharma: A Guide to Rapidly Accessing Transaction Insights
On January 8, 2024, Johnson & Johnson announced that it had reached a final agreement to acquire Ambrx Biopharma for $2 billion, advancing the development of the next generation of Antibody-Drug Conjugates (ADC). This acquisition grants Johnson & Johnson access to several of Ambrx Biopharma's ADC therapies, including ARX517, ARX788, ARX305, among others. Notably, ARX-517 is an ADC targeting Prostate-Specific Membrane Antigen (PSMA) for the treatment of metastatic castration-resistant prostate cancer (mCRPC) to meet the growing needs of over 185,000 patients suffering from mCRPC today.
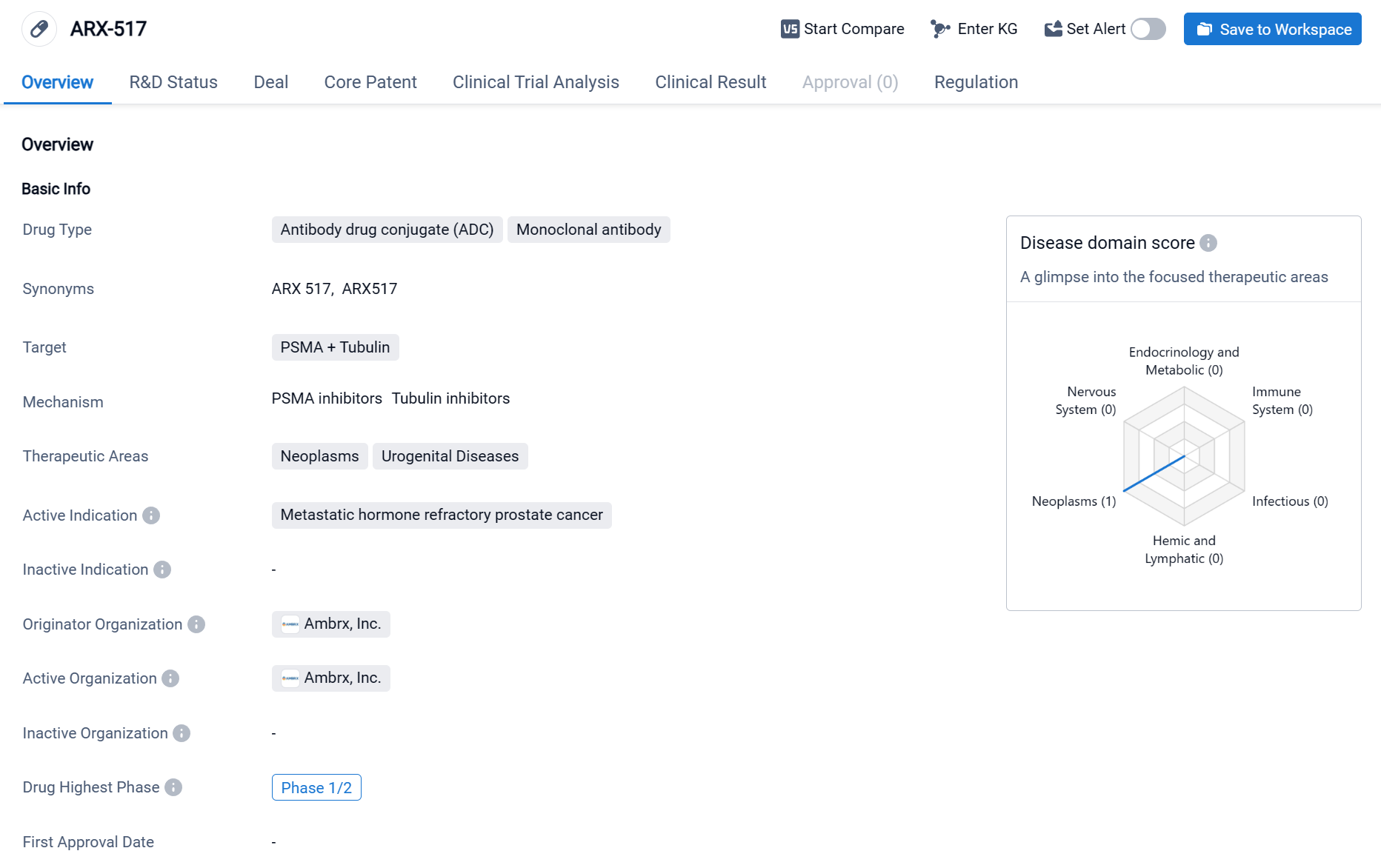
About ARX-517
ARX-517 is an antibody drug conjugate (ADC) and monoclonal antibody that targets PSMA (Prostate-Specific Membrane Antigen) and Tubulin. It falls under the therapeutic areas of neoplasms and urogenital diseases, specifically indicated for metastatic hormone refractory prostate cancer. Click the image below to directly embark on the exploration journey with the ARX-517!
In preclinical studies, the cancer cell killing payload of ARX517, pAF-AS269, is highly cytotoxic when delivered by a mAb into cancer cells. ARX517’s site-specific linkage, stable conjugation chemistry, and non-cleavable linker result in an ADC with a homogenous drug-antibody-ratio, mAb-like biophysical properties, and exceptional stability. Therefore, Ambrx believes ARX517 can promote highly specific tumor cell killing with minimal off-target toxicity. Currently, ARX-517 is in the highest phase of clinical development, which is Phase 1/2. The clinical result from NCT04662580 showed that following completion of the 21-day observation period at 3.4 mg/kg ARX-517, no dose limiting toxicities (DLTs) or serious adverse events (SAEs) were observed.
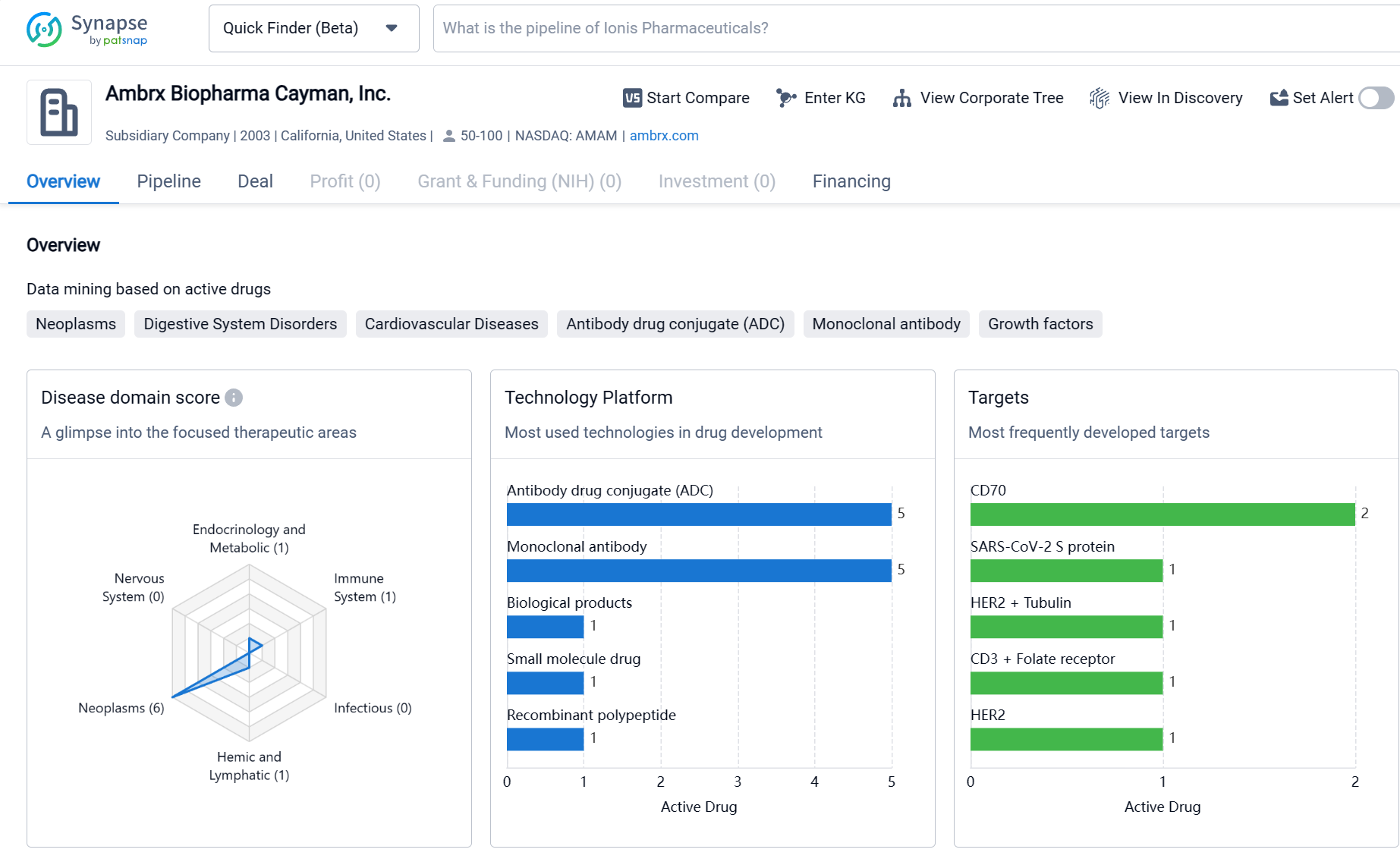
About Ambrx Biopharma
Ambrx Biopharma Cayman, Inc. is a biopharmaceutical organization based in California, United States, with a focus on biomedicine. The company has developed drugs in various therapeutic areas, with a significant emphasis on Neoplasms. The most frequently developed targets include CD70, SARS-CoV-2 S protein, and HER2 + Tubulin, among others. The pipeline analysis reveals ongoing research and development activities, with drugs in preclinical, clinical, and early-stage phases. Overall, Ambrx Biopharma Cayman, Inc. demonstrates a strong commitment to the development of innovative drugs for the treatment of various diseases, particularly cancer.
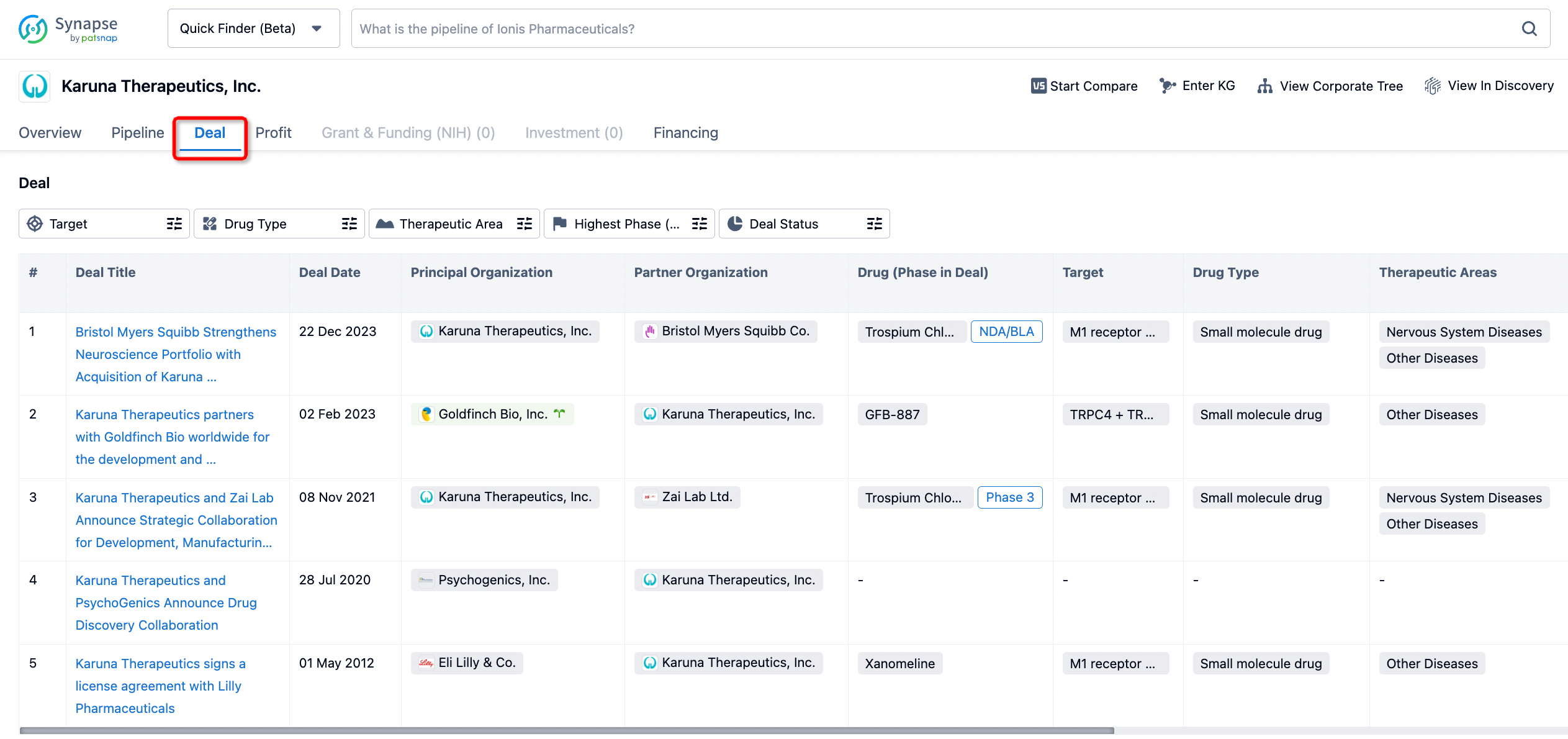
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
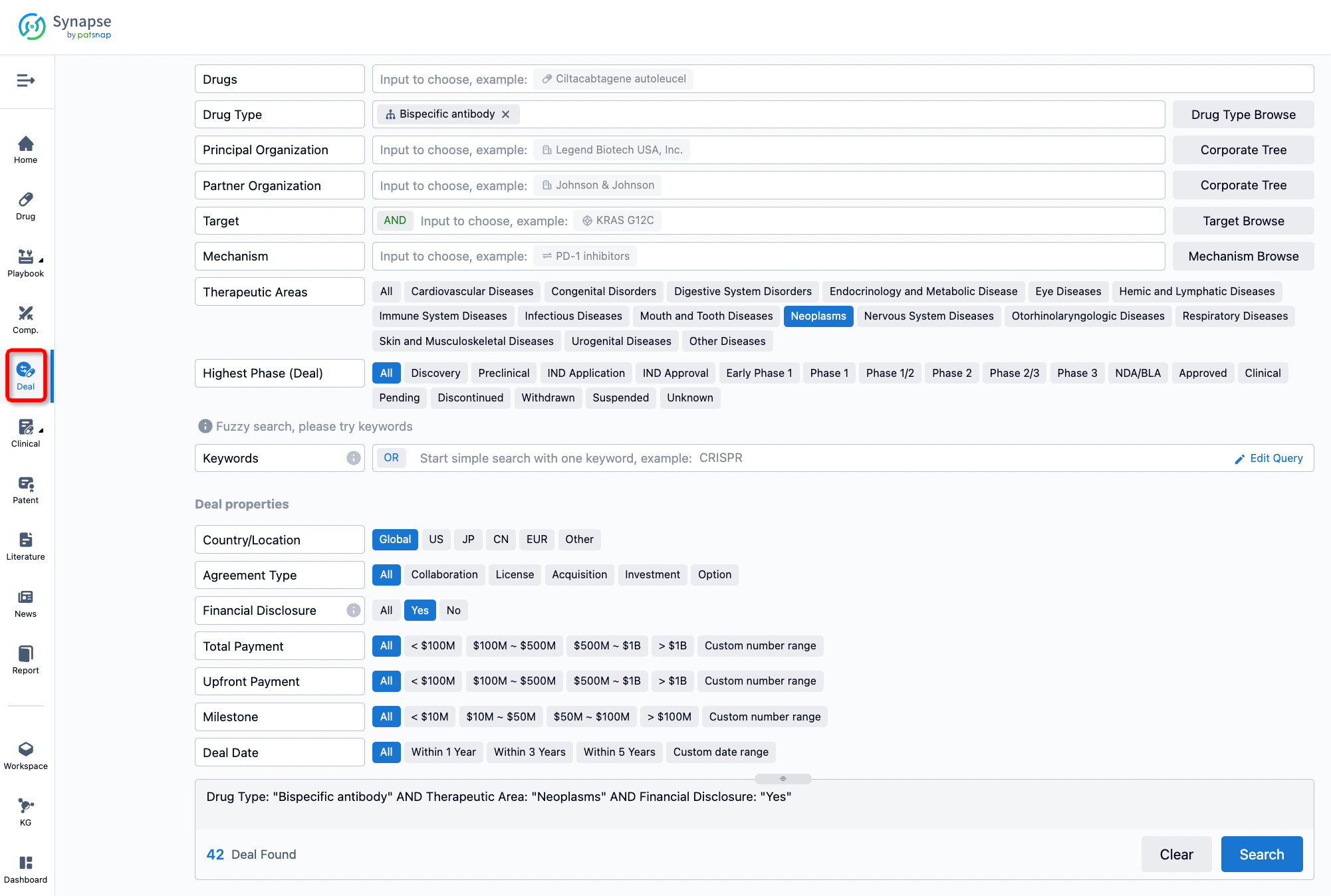
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
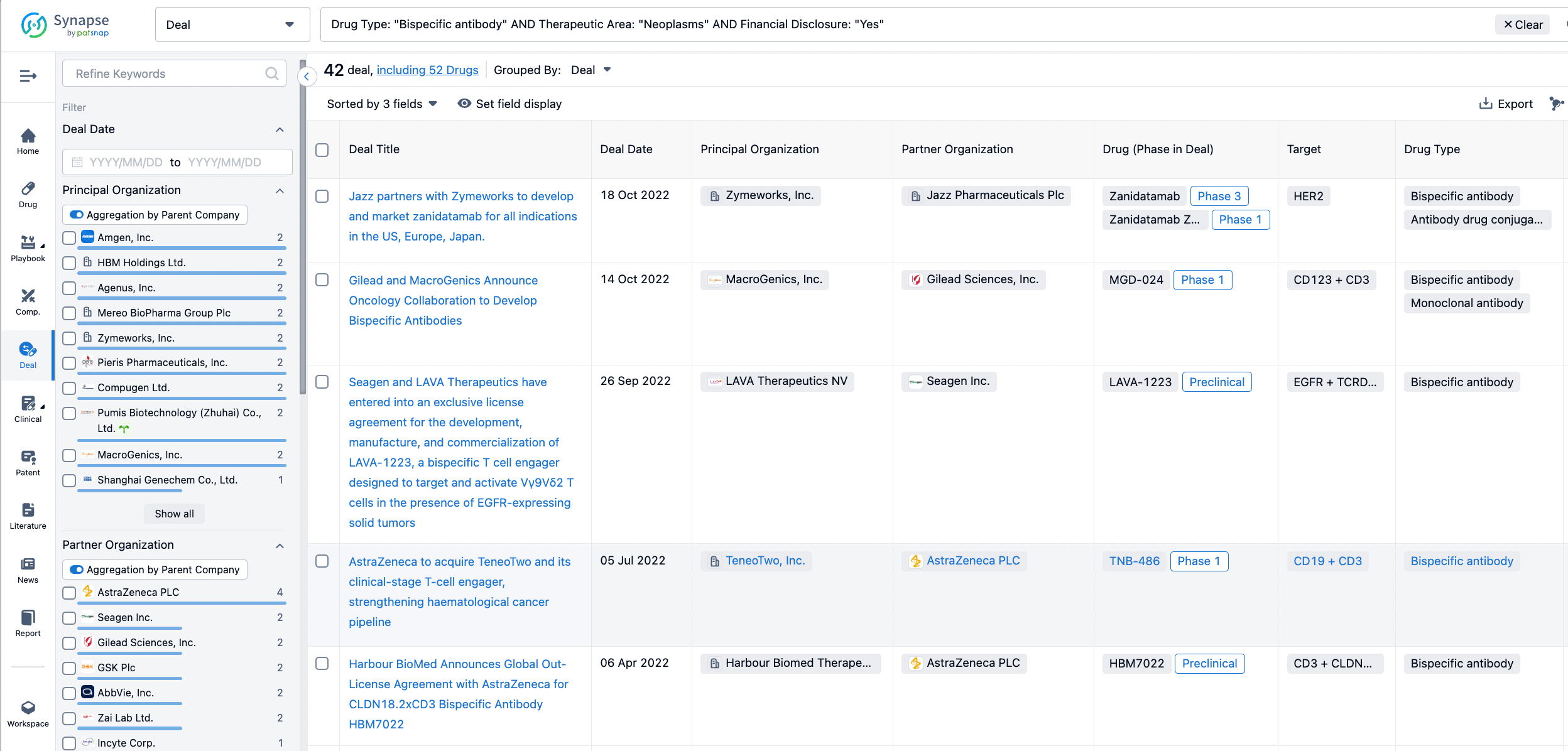
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
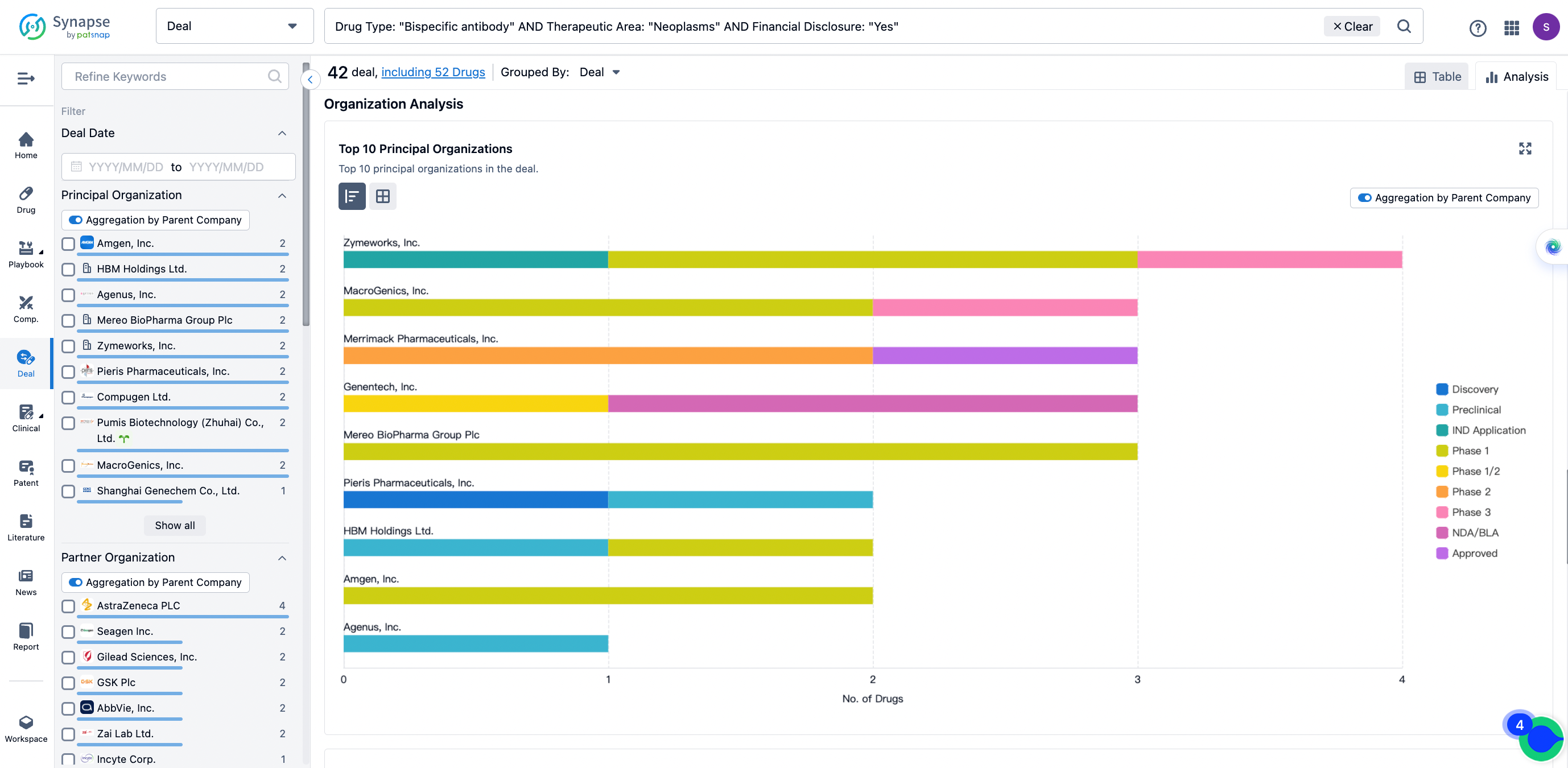
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
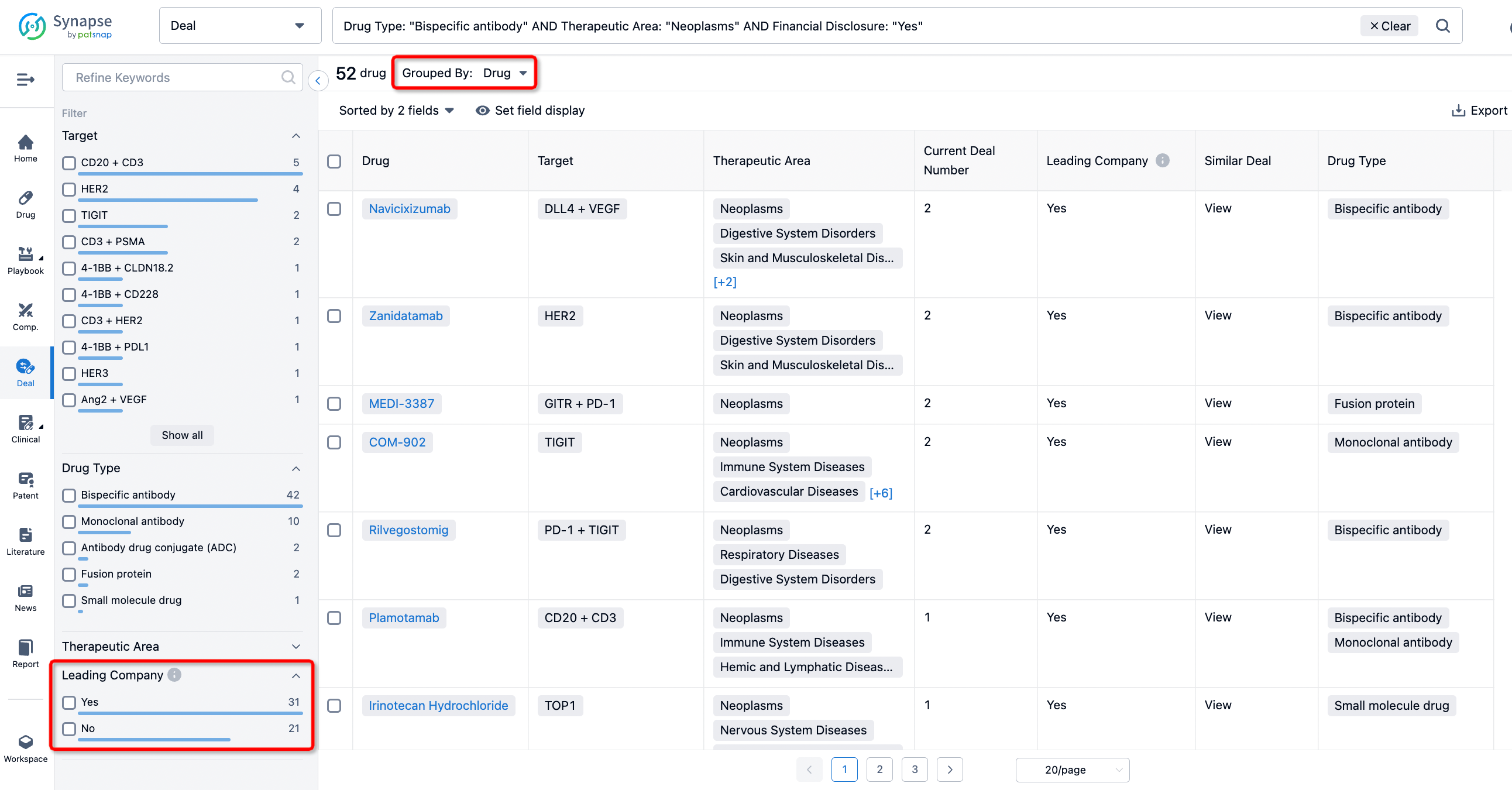
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
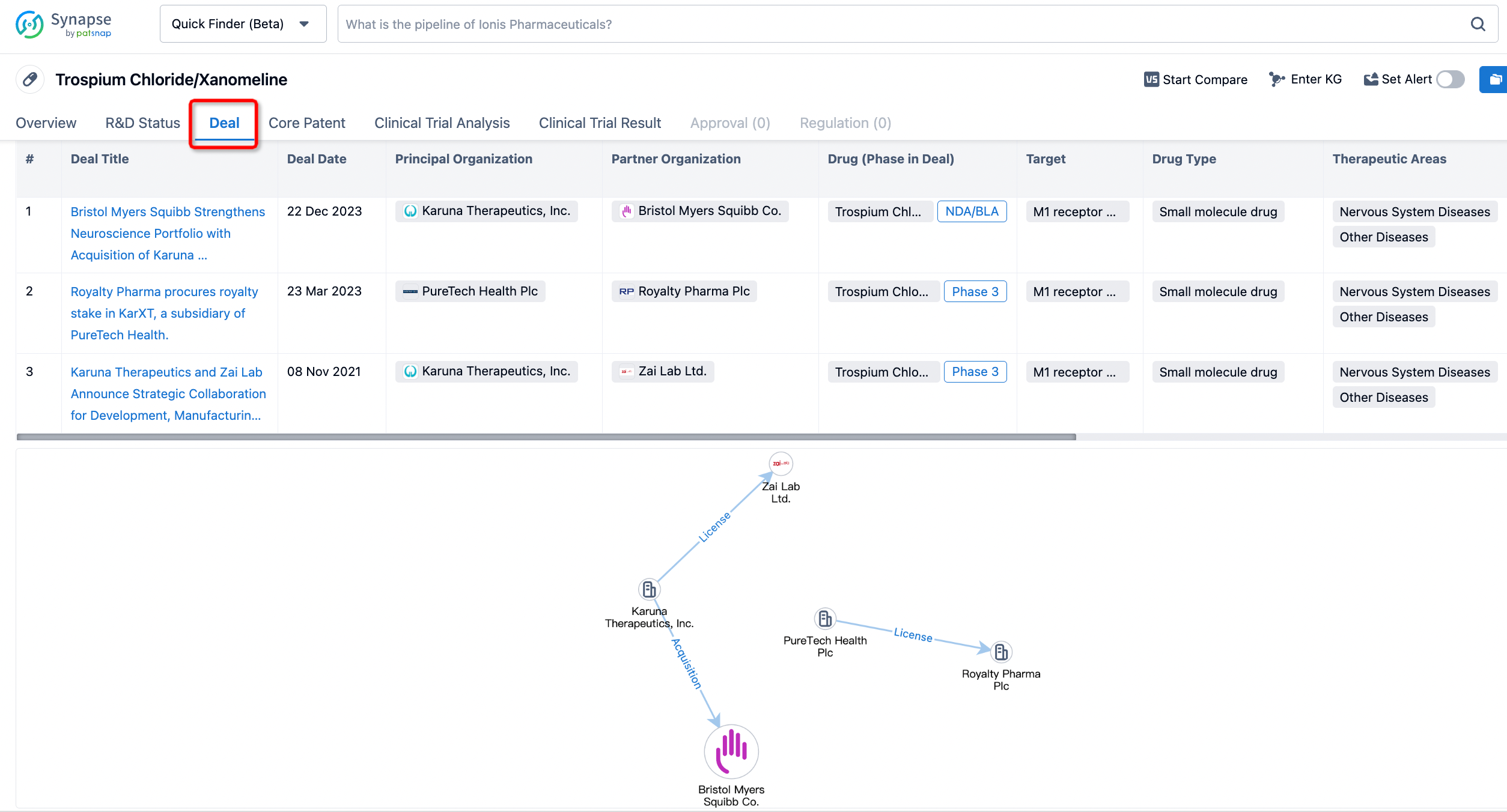
Click on the image below to embark on a brand new journey of drug discovery!