InnoCare Pharma announced FDA approval for its Bcl-2 inhibitor, ICP-248, to enter clinical trials
InnoCare Pharma, an enterprise operating at the commercial level within the biotechnology sector, has made public that its application for an Investigational New Drug concerning its B-cell lymphoma-2 (BCL2) inhibitor known as ICP-248 has been officially approved by the U.S. Food and Drug Administration. This marks the fifth pioneering pharmaceutical agent from InnoCare that has progressed to clinical trials on U.S. soil.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
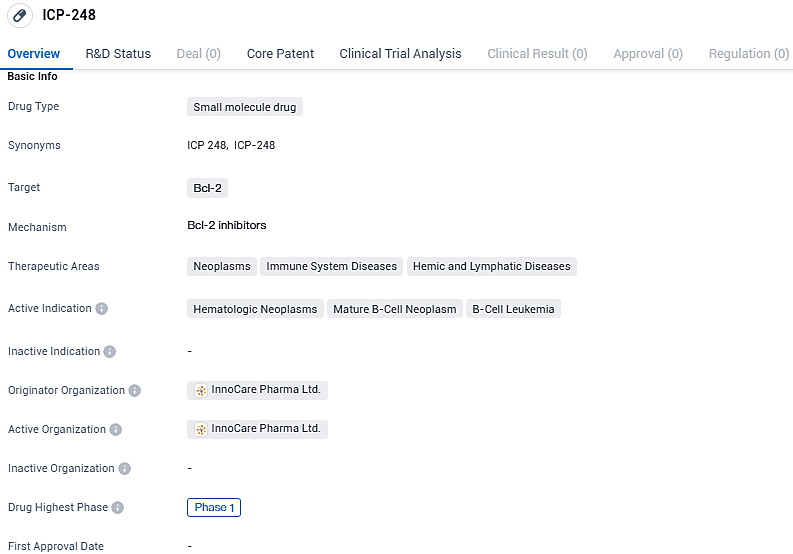
ICP-248, an innovative compound designed for oral administration, is a BCL2-specific antagonist that has been developed with the goal of managing blood cancers either as a stand-alone option or when used alongside additional treatments. The early-phase trial involving dosage increase is currently in progress within Chinese borders, with initial findings suggesting promising therapeutic and safety outcomes.
The protein BCL2 plays a significant role in controlling the cellular apoptosis pathway, and its overexpression has been linked to a myriad of blood cancer types. The therapeutic effects of ICP-248 stem from its ability to target BCL2 specifically, thereby reactivating the apoptotic process.
Speaking on behalf of InnoCare, Co-founder, Chairwoman, and CEO Dr. Jasmine Cui expressed her views by stating, "Following the introduction of orelabrutinib, ICP-248 is anticipated to be a key component of our global reach. Results from the on-going studies are expected to pave the way for using ICP-248 in the treatment of blood cancers, either alone or in combination with other modalities."
Dr. Cui further commented on the company's goals, "InnoCare is committed to establishing a dominant presence in the niche of hemato-oncology. As part of this commitment, we have been actively developing a diverse assortment of pharmaceutical candidates targeting crucial proteins in hemato-oncology including BTK, CD19, the bispecific CD20xCD3, BCL2, as well as E-3 ligase, all in a bid to meet the pressing health demands in this domain."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
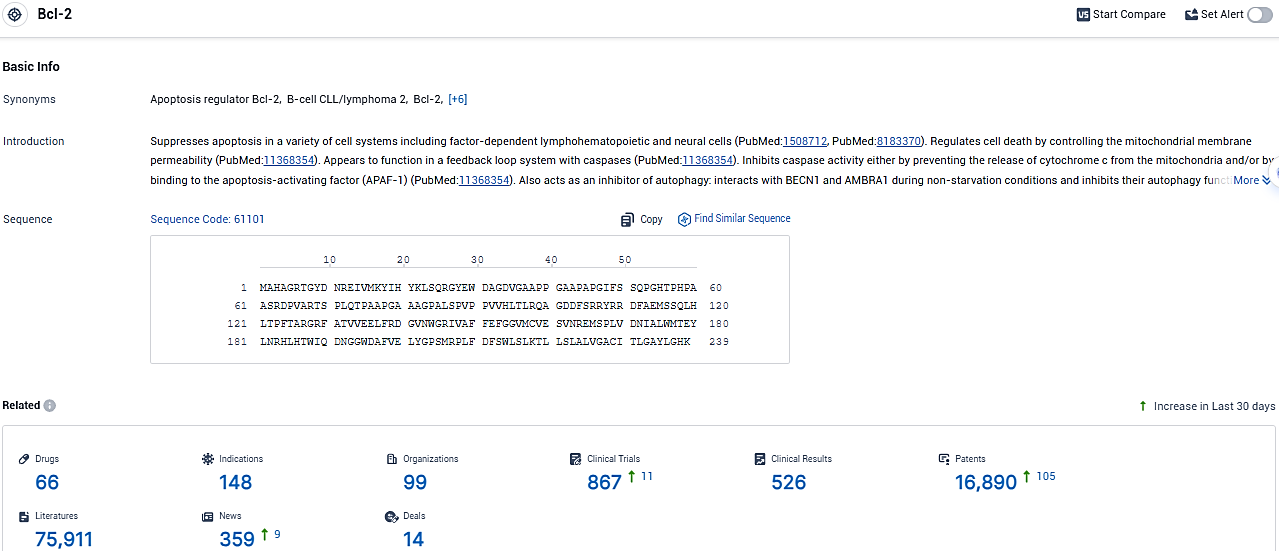
According to the data provided by the Synapse Database, As of January 19, 2024, there are 66 investigational drugs for the Bcl-2 target, including 148 indications, 99 R&D institutions involved, with related clinical trials reaching 867, and as many as 16890 patents.
ICP-248 targets Bcl-2 and is currently in Phase 1 of clinical trials for the treatment of hematologic neoplasms, mature B-cell neoplasms, and B-cell leukemia. While it is still in the early stages of development, the drug's potential to modulate cell survival pathways makes it an interesting candidate for further investigation in the field of biomedicine.