Progress in the Research of GnRHR Agonists
GnRHR, or Gonadotropin-Releasing Hormone Receptor, plays a crucial role in the human body's reproductive system. Located in the pituitary gland, GnRHR is responsible for binding to and responding to Gonadotropin-Releasing Hormone (GnRH). Upon activation, GnRHR stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland. These hormones are essential for the regulation of the menstrual cycle in females, as well as the production of testosterone in males. GnRHR's role in the body's endocrine system is vital for maintaining reproductive health and fertility.
GnRHR Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 81 GnRHR drugs worldwide, from 128 organizations, covering 53 indications, and conducting 1343 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.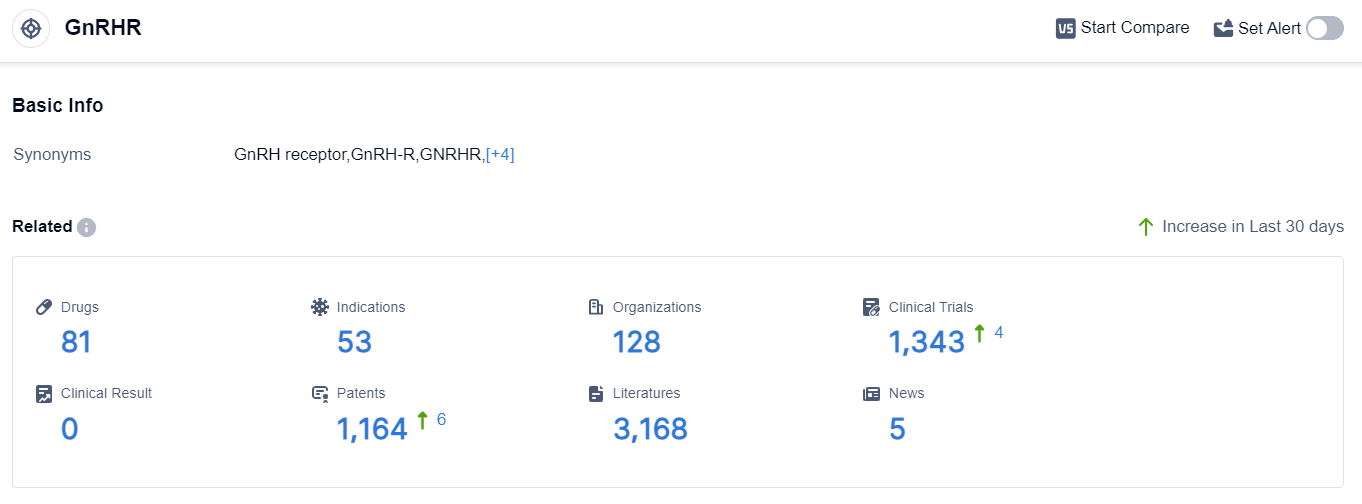
The analysis of the target GnRHR reveals a competitive landscape with multiple companies actively involved in the research and development of drugs. AbbVie, Inc., Sumitomo Chemical Co., Ltd., ObsEva SA, Ipsen SA, and Shanghai Livzon Pharmaceutical Co. Ltd. are the companies growing fastest under the current target.
The indications for the approved drugs targeting GnRHR cover a wide range of conditions related to the reproductive system and hormone regulation. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly, indicating intense competition around the innovative drugs.
The United States, European Union, Japan, China, and Norway are the countries/locations developing fastest under the current target, with China showing progress in the development of drugs for GnRHR. Overall, the target GnRHR presents a competitive landscape with diverse companies, indications, drug types, and countries/locations involved in research and development.
Key Drug: Leuprolide Acetate
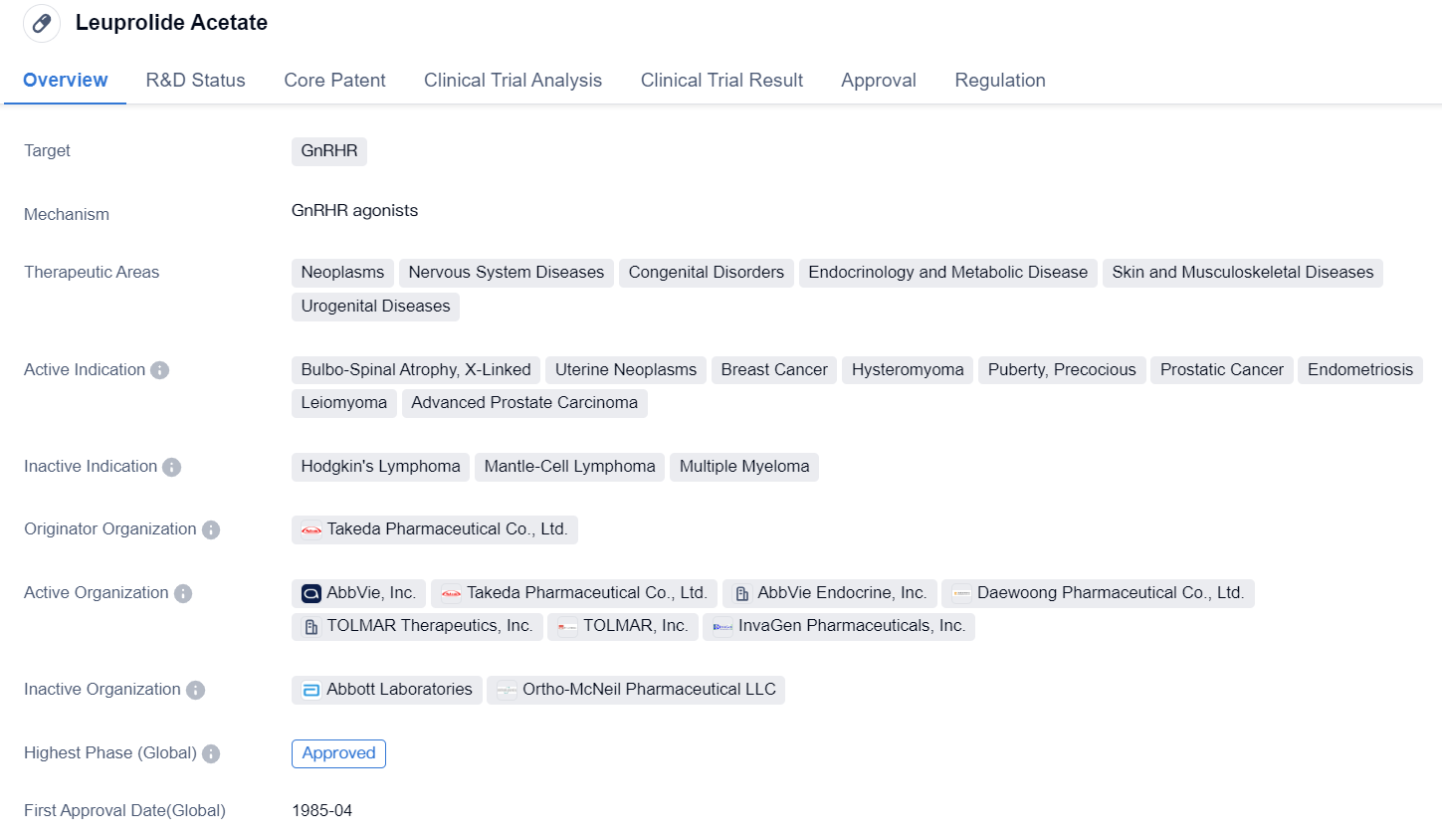
Leuprolide Acetate is a small molecule drug that targets the GnRHR. It has been approved for various therapeutic areas including neoplasms, nervous system diseases, congenital disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and urogenital diseases. The drug is indicated for the treatment of several conditions such as bulbo-spinal atrophy, X-linked, uterine neoplasms, breast cancer, hysteromyoma, puberty, precocious, prostatic cancer, endometriosis, leiomyoma, and advanced prostate carcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Leuprolide Acetate was first approved in the United States in April 1985 and is regulated as an orphan drug. The drug is developed by Takeda Pharmaceutical Co., Ltd., a renowned pharmaceutical organization. It has achieved the highest phase of approval both globally and in China.
Leuprolide Acetate's mechanism of action involves binding to the GnRHR, which leads to the suppression of gonadotropin secretion. This, in turn, inhibits the production of sex hormones such as estrogen and testosterone. By reducing the levels of these hormones, Leuprolide Acetate can effectively treat conditions related to hormone-dependent diseases.
The drug's approval for multiple therapeutic areas highlights its versatility and potential in treating various diseases. Its use in neoplasms suggests its efficacy in combating cancerous growths, while its application in nervous system diseases and congenital disorders indicates its potential in addressing neurological and developmental conditions. Additionally, its approval for endocrinology and metabolic diseases suggests its effectiveness in regulating hormonal imbalances.
Leuprolide Acetate's long history of approval since 1985 demonstrates its established safety and efficacy profile. Being regulated as an orphan drug further emphasizes its importance in treating rare diseases and conditions. The drug's originator, Takeda Pharmaceutical Co., Ltd., is a reputable organization known for its contributions to the pharmaceutical industry.
In conclusion, Leuprolide Acetate is a small molecule drug that targets the GnRHR and has been approved for various therapeutic areas. Its indications cover a wide range of conditions, including cancer, hormonal disorders, and developmental abnormalities. With its long history of approval and regulation as an orphan drug, Leuprolide Acetate holds promise in the field of biomedicine.
Goserelin Acetate
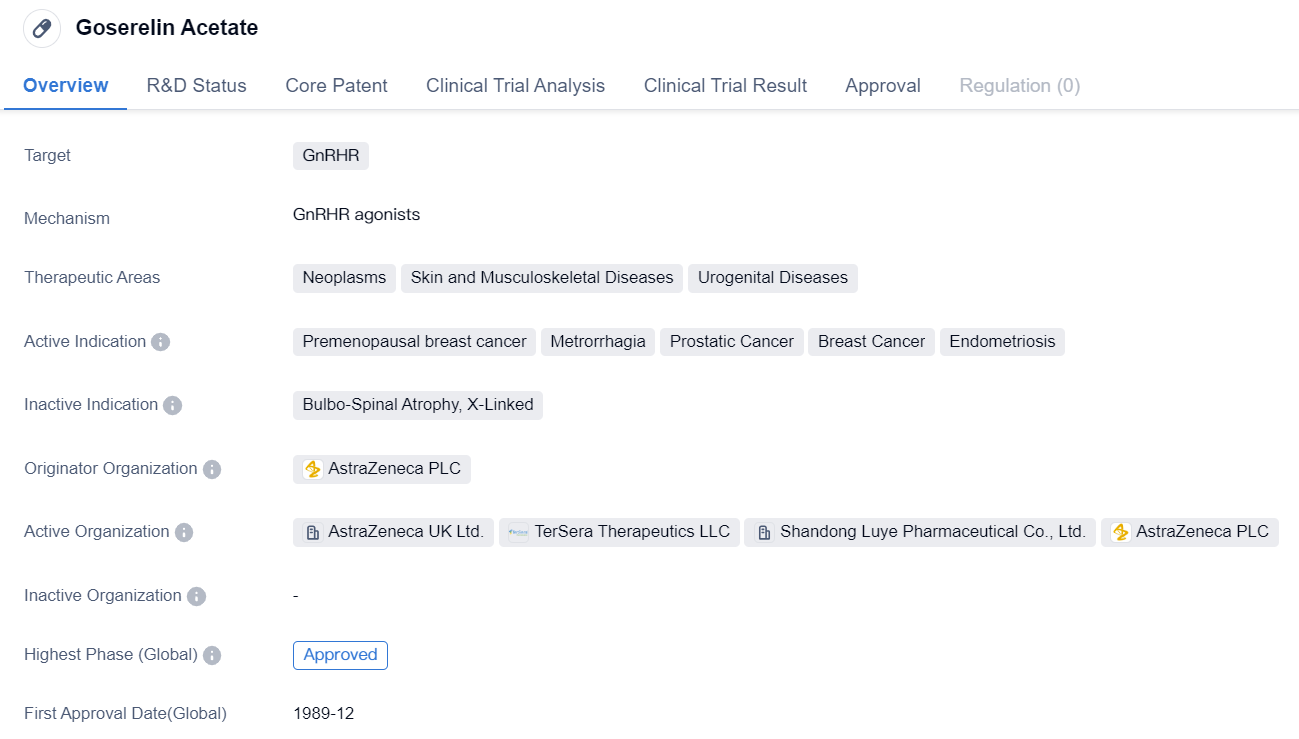
Goserelin Acetate is a small molecule drug that targets the GnRHR. It is primarily used in the treatment of various neoplasms, skin and musculoskeletal diseases, and urogenital diseases. The drug has been approved for the treatment of premenopausal breast cancer, metrorrhagia (abnormal uterine bleeding), prostatic cancer, breast cancer, and endometriosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug was developed by AstraZeneca PLC, a pharmaceutical company known for its expertise in the field of biomedicine. Goserelin Acetate has received approval for its highest phase of development both globally and in China, indicating its efficacy and safety profile. The drug was first approved for use in the United States in December 1989.
Goserelin Acetate works by inhibiting the release of gonadotropin-releasing hormone (GnRH), which in turn suppresses the production of sex hormones such as estrogen and testosterone. This mechanism of action makes it particularly effective in the treatment of hormone-sensitive cancers, including breast and prostate cancer.
Premenopausal breast cancer is a type of breast cancer that occurs in women before they reach menopause. Goserelin Acetate is used in combination with other therapies to suppress the production of estrogen, which fuels the growth of breast cancer cells. Metrorrhagia refers to abnormal uterine bleeding that occurs between menstrual periods. Goserelin Acetate can help regulate menstrual bleeding by suppressing the production of hormones that control the menstrual cycle.
Prostatic cancer, also known as prostate cancer, is a type of cancer that affects the prostate gland in men. Goserelin Acetate is used to reduce the production of testosterone, which can slow down the growth of prostate cancer cells. Endometriosis is a condition in which the tissue lining the uterus grows outside of it, causing pain and fertility problems. Goserelin Acetate can help manage endometriosis by suppressing the production of estrogen, which promotes the growth of endometrial tissue.
In summary, Goserelin Acetate is a small molecule drug developed by AstraZeneca PLC that targets the GnRHR. It has been approved for the treatment of various neoplasms, skin and musculoskeletal diseases, and urogenital diseases. The drug is particularly effective in the treatment of premenopausal breast cancer, metrorrhagia, prostatic cancer, breast cancer, and endometriosis. Its mechanism of action involves suppressing the production of sex hormones, making it suitable for hormone-sensitive conditions. Goserelin Acetate has been approved for use globally since 1989 and has shown promising results in clinical trials.