Coya Therapeutics' COYA 302 Exhibits Anti-Inflammatory Effects in Brain with Subcutaneous Dose in Parkinson's Mouse Model
Coya Therapeutics, Inc. (NASDAQ: COYA) (“Coya”), a biotechnology firm at the clinical stage focused on creating biologics to improve regulatory T cell (Treg) function, has revealed that COYA 302, when administered subcutaneously, exhibits a direct anti-inflammatory impact on the CNS in a preclinical mouse model related to inflammation in Parkinson’s Disease (PD).
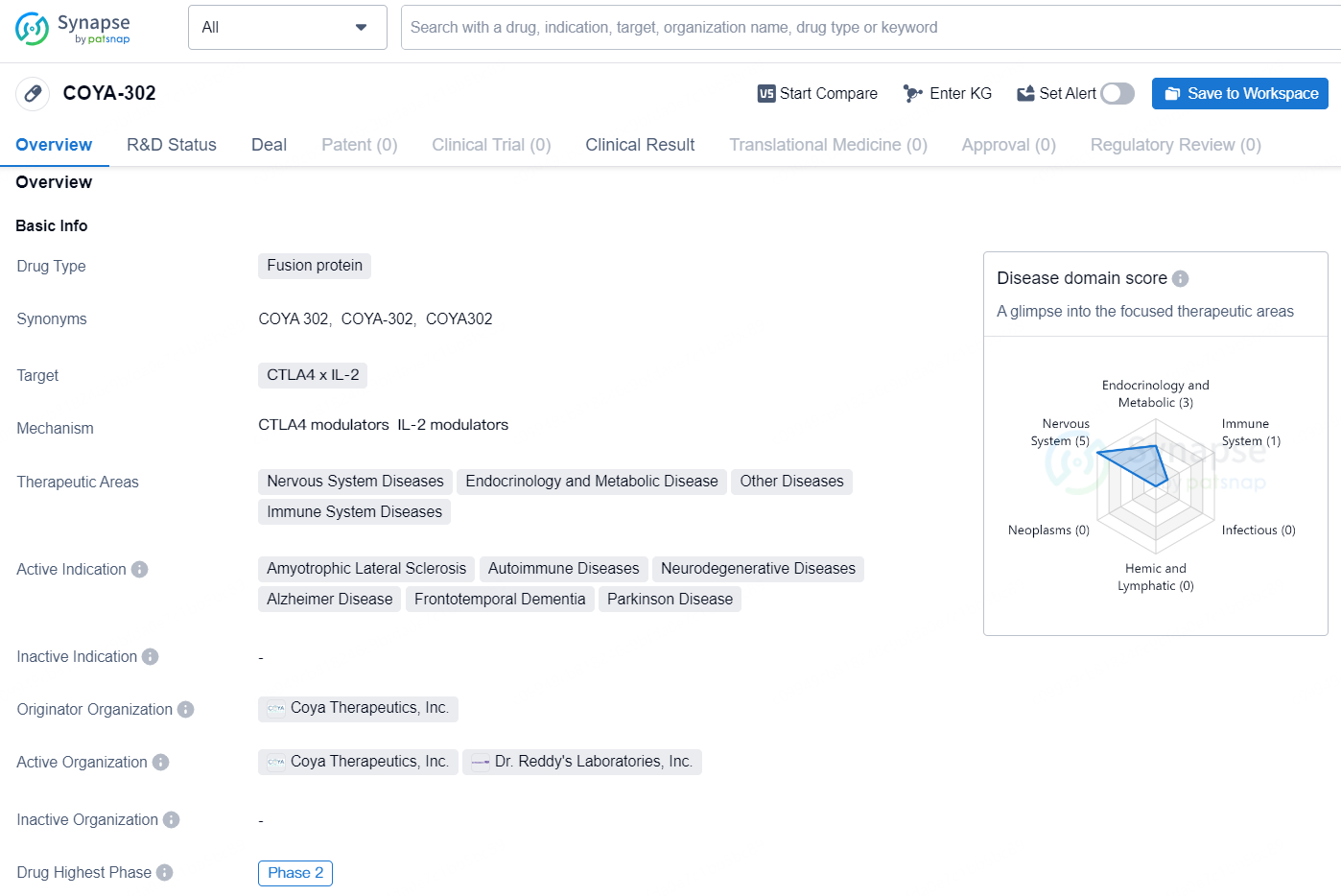
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Dr. Arun Swaminathan, Coya's Chief Business Officer and soon-to-be Chief Executive Officer, remarked: "We are confident that the efficacy of peripherally administered biologics (COYA 302) in significantly alleviating brain inflammation offers strategies not just for controlling neuro-inflammation in PD but also for other inflammation-driven neurodegenerative disorders such as Alzheimer’s disease (AD) and Frontotemporal Dementia (FTD)."
Parkinson's disease is marked by the targeted loss of dopaminergic neurons in brain areas crucial for motor control (nigrostriatal pathway). Presently, inflammation and immune dysfunction due to the related decline in systemic Treg function are acknowledged as key disease mediators and contributors to PD progression. Addressing the ongoing pro-inflammatory mechanisms worsening the disease, and boosting immunosuppressive cells like Tregs, might offer disease-modifying benefits for PD patients.
In an inflammatory mouse model of PD, subcutaneous COYA 302 injections significantly reduced inflammation and microglial activation in the nigrostriatal brain regions, specifically the dorsal striatum and substantia nigra, which are vital for motor control. Microglial activation is a crucial factor in PD pathology and neurodegeneration. Inhibiting microglial activity might be a promising therapeutic approach to delay PD progression. Furthermore, subcutaneous COYA 302 injections led to a decrease in astrocyte count and activity (astrogliosis) in the nigrostriatal pathway. Pathogenic astrocyte activation contributes to neurodegeneration in PD, suggesting that mitigating this activation could be another therapeutic target. The direct impact of COYA 302 in reducing neuroinflammatory elements known to facilitate neurodegeneration is encouraging and merits further clinical exploration in preclinical models and eventual patient trials. The Company plans to present and/or publish these findings in a peer-reviewed context.
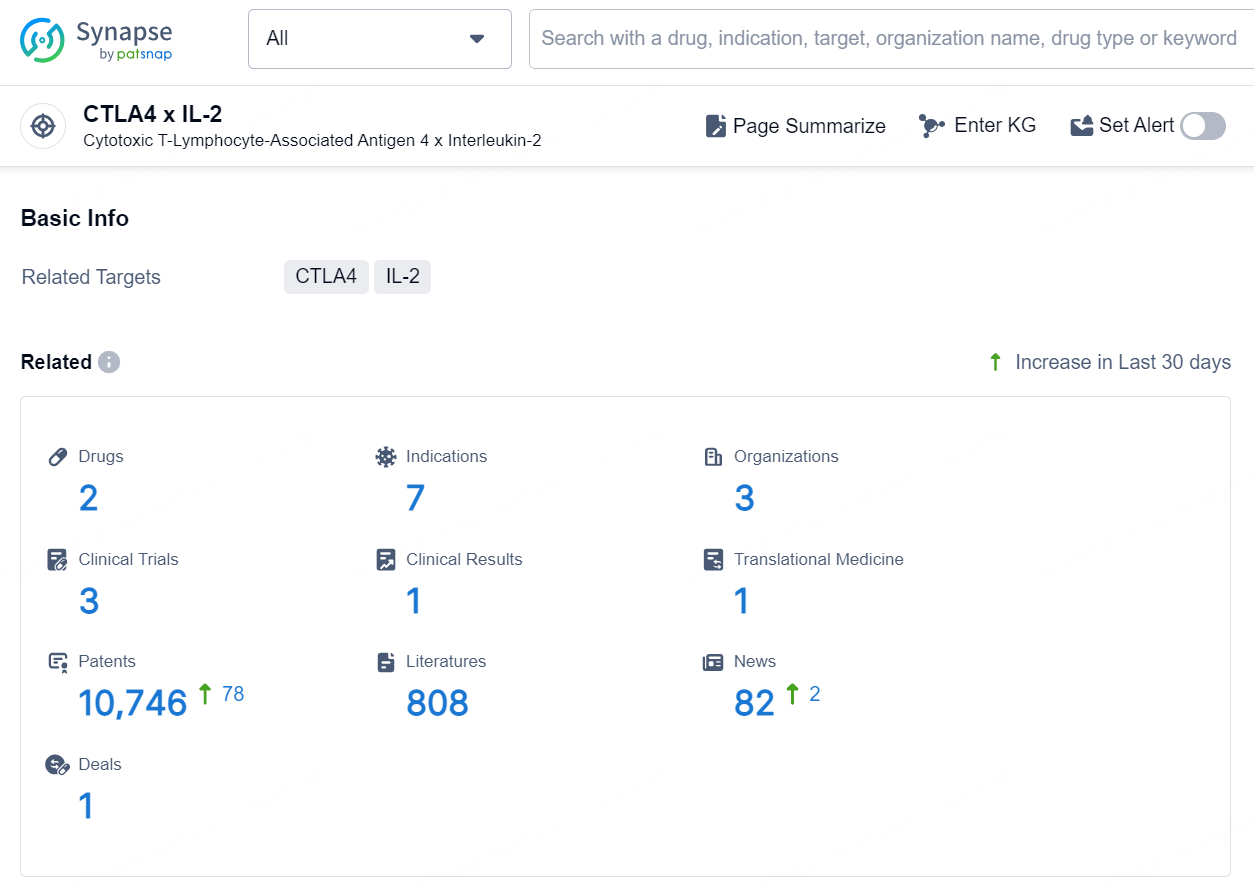
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 2 investigational drugs for the CTLA4 x IL-2 target, including 7 indications, 3 R&D institutions involved, with related clinical trials reaching 3, and as many as 10746 patents.
COYA-302 is a fusion protein drug that targets CTLA4 x IL-2 and falls under the therapeutic areas of Nervous System Diseases, Endocrinology and Metabolic Disease, Other Diseases, and Immune System Diseases. The active indications for this drug include Amyotrophic Lateral Sclerosis, Autoimmune Diseases, Neurodegenerative Diseases, Alzheimer Disease, Frontotemporal Dementia, and Parkinson Disease. The drug is currently in Phase 2 of development and is being developed by Coya Therapeutics, Inc.