FDA Approves TREMFYA® for Severe Ulcerative Colitis, Enhancing Johnson & Johnson's Market Position
Johnson & Johnson (NYSE: JNJ) has announced that the U.S. Food and Drug Administration (FDA) has given approval to TREMFYA® (guselkumab) for use in treating adults with moderate to severe ulcerative colitis (UC), a long-term condition where the colon's lining becomes inflamed. TREMFYA® stands out as the first and solely approved fully-human, dual-function monoclonal antibody targeting IL-23 and simultaneously binding to CD64, a receptor located on cells responsible for producing IL-23. IL-23 is a cytokine emitted by activated monocytes/macrophages and dendritic cells, which is recognized as a contributing factor to immune-mediated diseases, including UC.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"TREMFYA therapy led to a notable enhancement in the chronic symptoms of ulcerative colitis, significantly normalizing the endoscopic view of the intestinal lining," stated David T. Rubin, MD, Director of the Inflammatory Bowel Disease Center at the University of Chicago Medicine, and principal investigator of the QUASAR study. "The recent approval of TREMFYA leverages its established clinical and safety profile as an IL-23 inhibitor, representing a key advancement in managing this persistent inflammatory condition."
The approval of TREMFYA for UC is based on results from the ongoing Phase 2b/3 QUASAR trial, which assesses the drug's effectiveness and safety in adult patients with moderately to severely active ulcerative colitis who have not responded adequately to standard treatments, other biologics, and/or JAK inhibitors. Key findings from the QUASAR trial include:
At week 44, 50% of patients receiving 200 mg TREMFYA subcutaneously every four weeks, and 45% receiving 100 mg every eight weeks, achieved clinical remission, compared to 19% of those given a placebo (p<0.001).
34% and 35% of patients on 200 mg and 100 mg doses, respectively, achieved endoscopic remission at one year compared to 15% of those on placebo (p<0.001).
"There is an urgent need for new UC treatments that significantly enhance symptom relief and offer the potential for remission, including both overall clinical remission and visible healing of the colon through endoscopic remission," commented Christopher Gasink, MD, Vice President of Medical Affairs for Gastroenterology & Autoantibody at Johnson & Johnson Innovative Medicine. "TREMFYA has shown high endoscopic remission rates after one year of treatment in the QUASAR trial, setting a new efficacy benchmark for this inflammatory bowel disease."
For ulcerative colitis treatment, TREMFYA is given as a 200 mg intravenous induction dose at weeks zero, four, and eight by a healthcare provider. The recommended maintenance dose is either 100 mg subcutaneously at week 16 and subsequently every eight weeks, or 200 mg subcutaneously at week 12 and then every four weeks. The maintenance dose can be self-administered or given by a caregiver after appropriate training. It is advised to use the lowest effective dose to maintain a therapeutic response.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
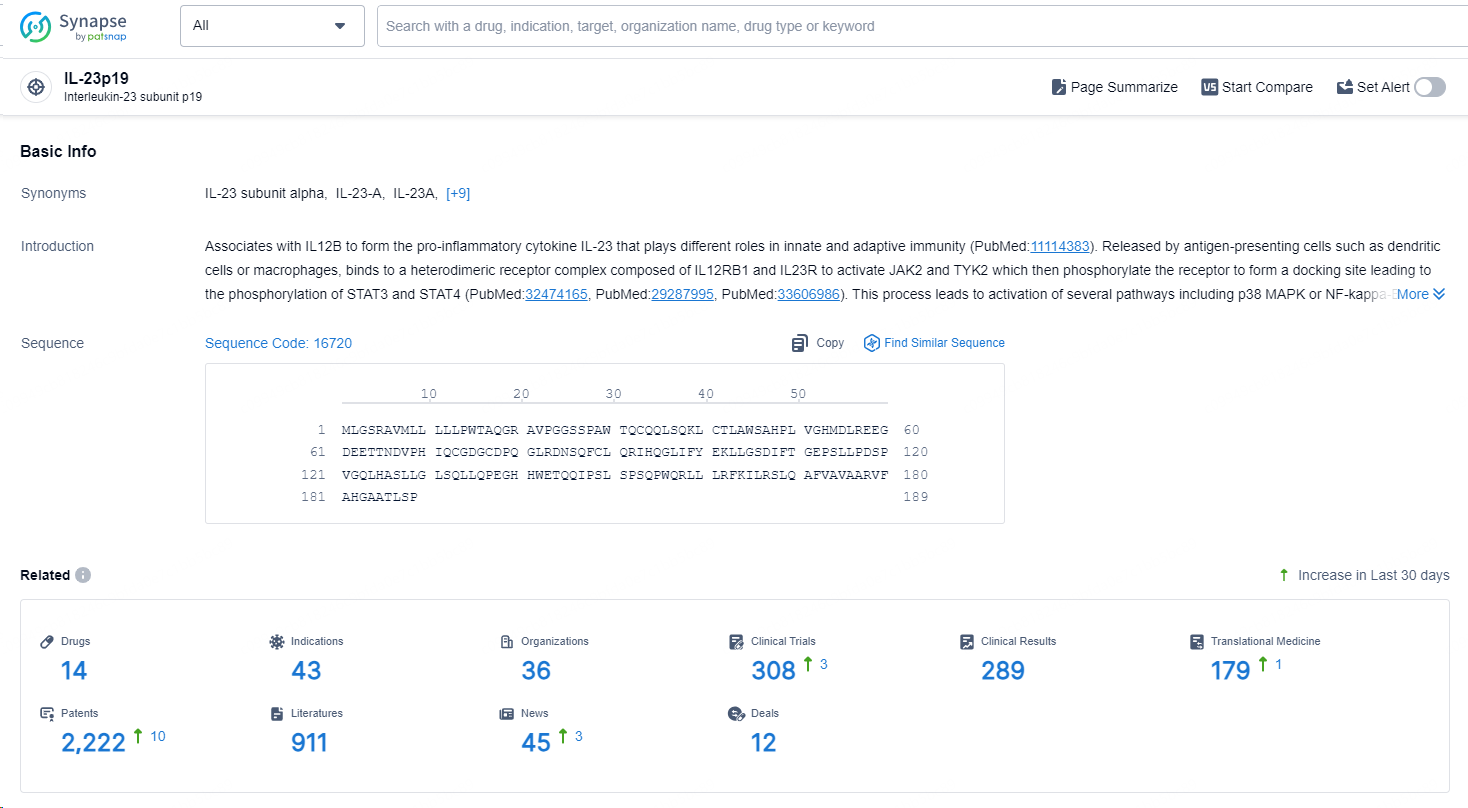
According to the data provided by the Synapse Database, As of September 14, 2024, there are 14 investigational drug for the IL-23p19 targets, including 43 indications, 36 R&D institutions involved, with related clinical trials reaching 308, and as many as 2222 patents.
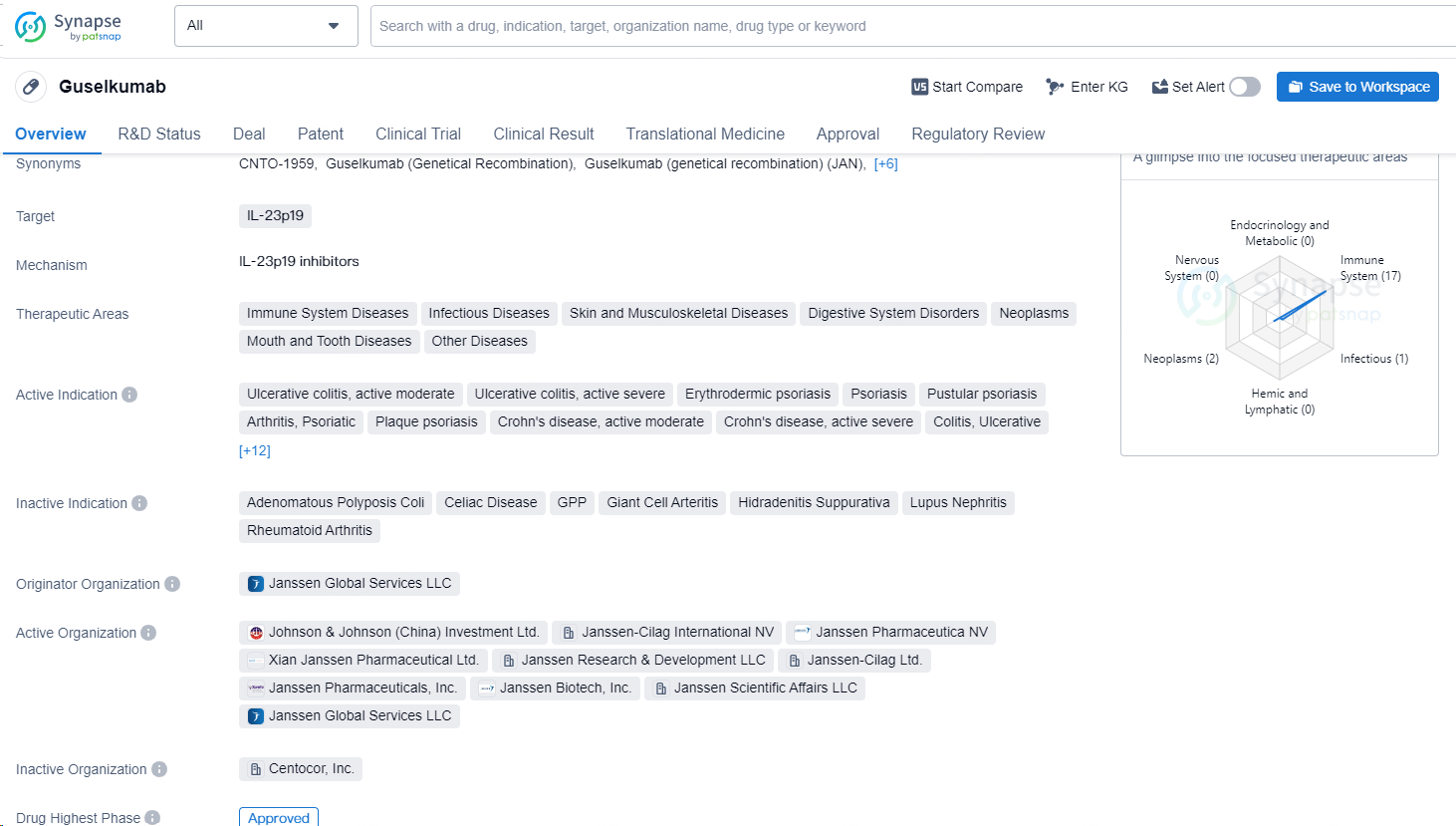
Guselkumab is a monoclonal antibody drug that targets IL-23p19 and is used in the treatment of various immune system diseases, infectious diseases, skin and musculoskeletal diseases, digestive system disorders, neoplasms, mouth and tooth diseases, as well as other diseases. The drug is indicated for ulcerative colitis (moderate and severe), erythrodermic psoriasis, psoriasis, pustular psoriasis, psoriatic arthritis, plaque psoriasis, Crohn's disease (moderate and severe), ulcerative colitis, Crohn disease, pediatric Crohn's disease, salivary gland adenoma, pleomorphic, perianal fistula due to Crohn's disease, juvenile idiopathic arthritis, scalp psoriasis, chronic large plaque psoriasis, neoplasms, pyoderma gangrenosum, wounds and injuries, systemic scleroderma, and pediatric ulcerative colitis.