QL Biopharm Shared Phase 1c Study Findings on New Monthly GLP-1RA ZT002 at EASD 2024
Beijing QL Biopharmaceutical Co., Ltd., a clinical-stage biopharmaceutical organization focused on the creation of innovative biologic treatments for metabolic diseases, revealed Phase 1c clinical results for its primary drug candidate ZT002 at the European Association for the Study of Diabetes (EASD) Annual Meeting 2024, held in Madrid, Spain.
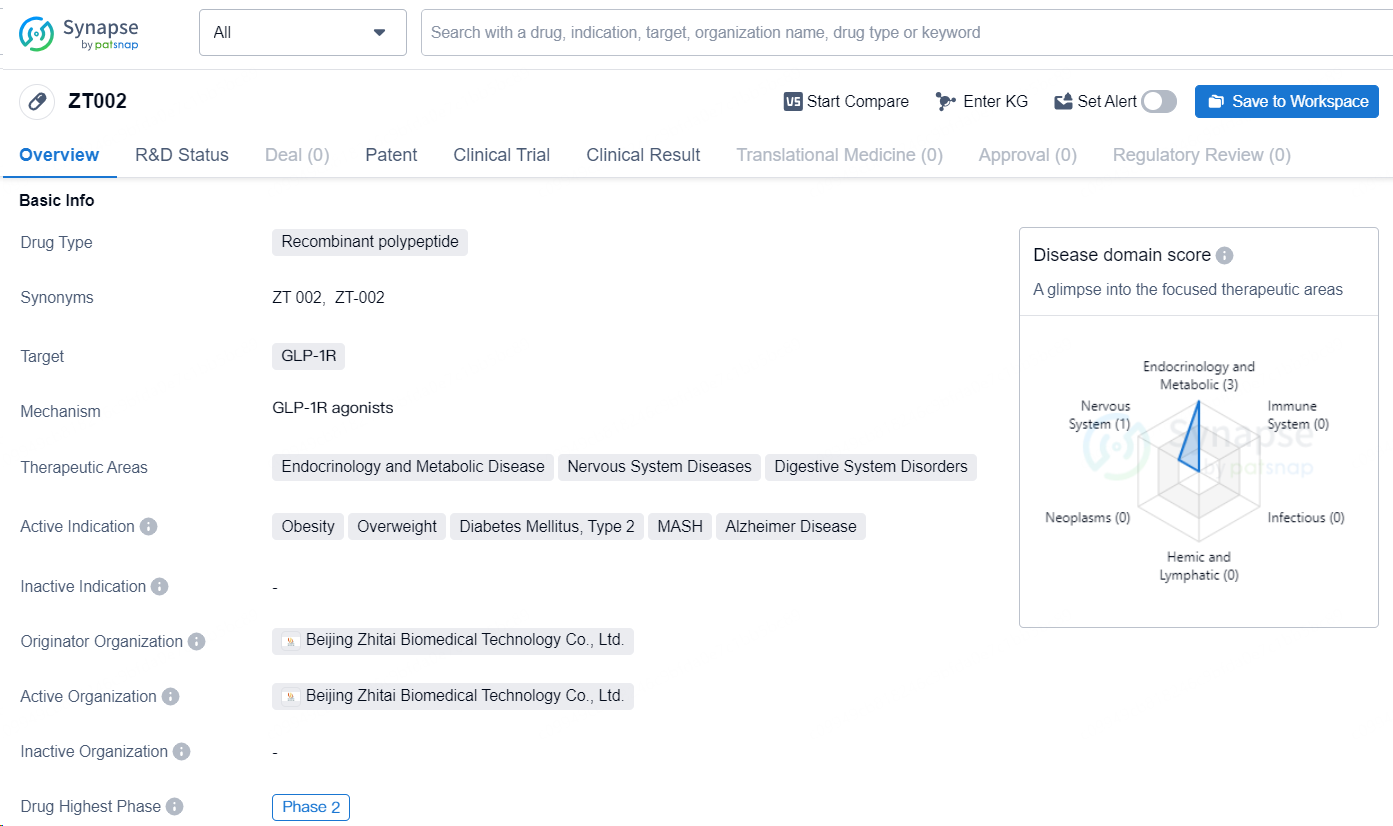
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The presentation titled ‘ZT002, a novel ultra long-acting GLP-1 receptor agonist in adults with overweight or obesity: A randomized, placebo-controlled, multiple ascending dose phase 1c study’ detailed recent findings on QL Biopharm’s leading drug ZT002 – an innovative ultra long-acting glucagon-like peptide-1 receptor agonist (GLP-1RA) designed for treating obesity with monthly subcutaneous dosing.
The study included 28 overweight/obese participants in its initial segment, Part A, divided into two cohorts. Participants eligible for the study were randomly assigned to receive biweekly (Q2W) subcutaneous injections of ZT002 (dose escalated to 40 mg or 80 mg; n=10+10) or a matching placebo (n=4+4) over a treatment duration of 12 or 14 weeks, respectively, for the 40 mg and 80 mg dose groups. The principal outcomes focused on safety and tolerability, while secondary outcomes encompassed pharmacokinetics, pharmacodynamics, and immunogenicity. All outcomes were successfully achieved. A 6-week washout period followed for pharmacokinetic assessment before proceeding to an open-label extension (Part B).
During this extension phase, patients from the ZT002 80 mg Q2W cohort volunteered for an additional 12-week treatment phase involving a once-monthly (Q4W) administration of ZT002 at 120 mg (n=8). The primary outcomes again focused on safety and tolerability, with secondary outcomes being pharmacokinetics, pharmacodynamics, and immunogenicity. These endpoints were also successfully achieved.
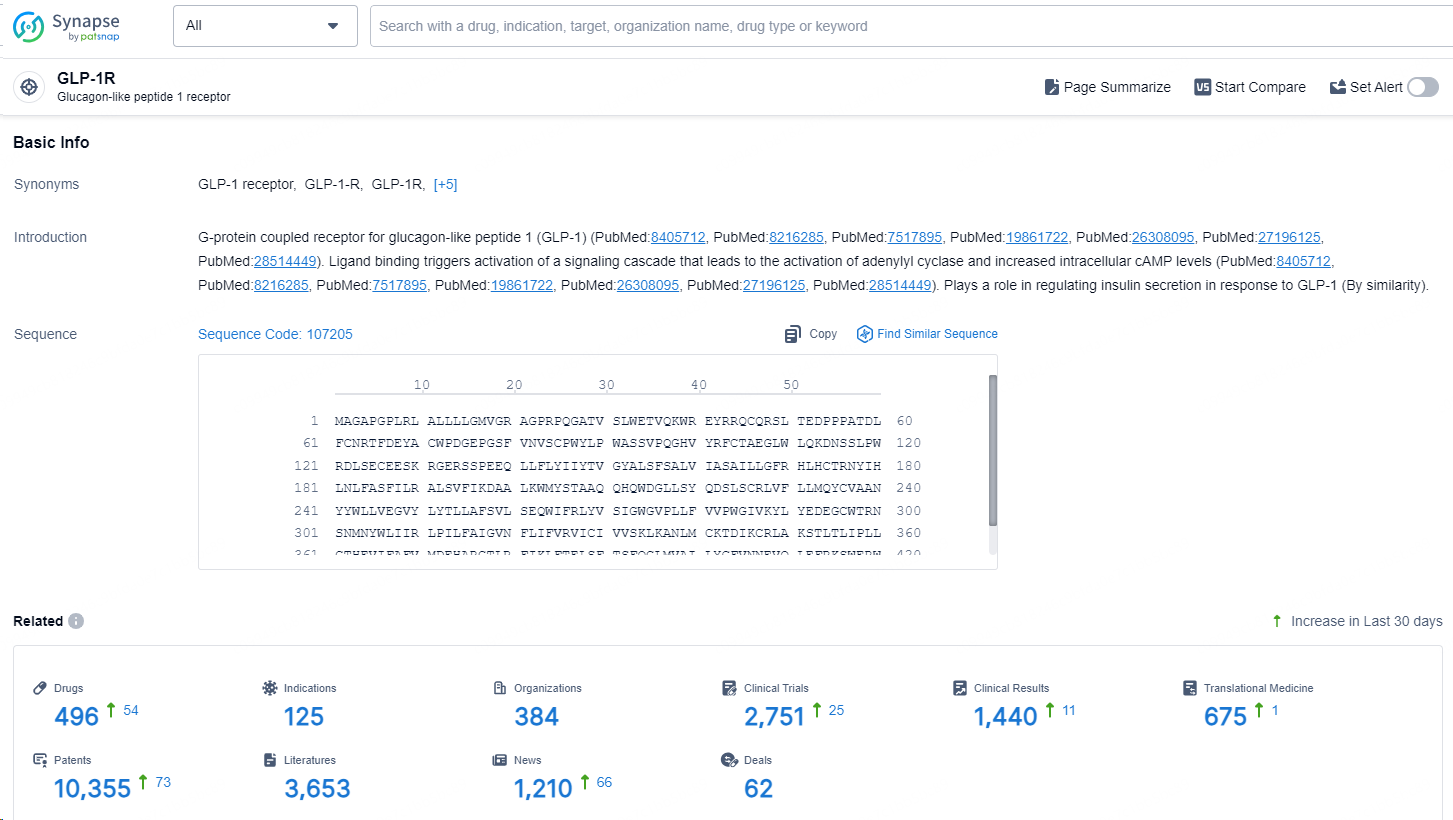
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 14, 2024, there are 496 investigational drug for the GLP-1R targets, including 125 indications, 384 R&D institutions involved, with related clinical trials reaching 2751, and as many as 10355 patents.
ZT002 is a recombinant polypeptide drug developed by Beijing Zhitai Biomedical Technology Co., Ltd. The drug targets the GLP-1R and is designed to address a range of therapeutic areas including endocrinology and metabolic disease, nervous system diseases, and digestive system disorders. Specifically, it is indicated for the treatment of obesity, overweight, diabetes mellitus type 2, nonalcoholic steatohepatitis (NASH), and Alzheimer's disease.